Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Even the best-designed devices, prepared with careful simulations and usability studies, can behave very differently when used in actual clinical or emergency situations.
-

In this Before the Build episode, Eric Olson and Paul Charlebois dive into the importance of organ transplant logistics when designing effective medical devices.
-

In this Before the Build episode, Eric Olson and Paul Charlebois reflect on the value of patient-centered field research—and how firsthand observation can reframe design priorities and impact outcomes in profound ways.
-

What does empathetic medical design really look like in practice? Eric and Paul discuss how emotional insight from field research can profoundly impact the design of medical devices
-
Many clients now request their devices to look and feel like Apple products. But achieving that level of simplicity and elegance is not as easy as it seems.
-

Paul Charlebois and Eric Olson explore how contextual inquiry in MedTech drives smarter product design. By observing how users interact with devices in real settings, product teams can gather early insights that shape usability, adoption, and safety—long before development begins.
-

There are a lot of things to consider when creating a summative usability test. The first time can be overwhelming. What scenarios do I test? How do I identify a critical task? How many participants do I need? Do I need to test the training? In this eGuide I will help you avoid common mistakes, like using a formative test structure for a summative usability test. I will also provide some tricks and tips to help you get the most out of your test participants and testing.
-
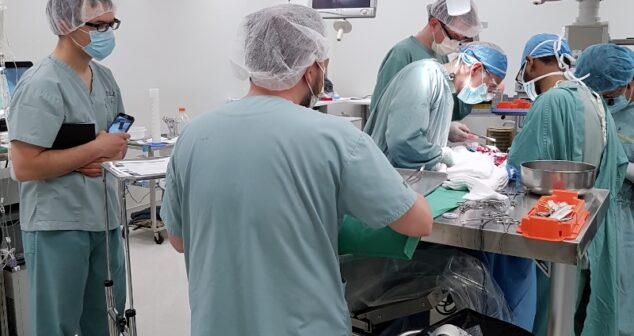
Procedural observations of medical procedures are invaluable and inform designs with perspective of users, procedural workflow and intended use environment.