Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Developing medical devices with software involves a wide-ranging set of unique challenges. Our software engineering team offer advice on offered advice on software in medical devices, FDA, Software as a Medical Device (SaMD), risks, cybersecurity, and Open Source software.
-

SBOM Analysis and Value covers the FDA “Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions guidance, role of Software Bills of Materials (SBOMs), and how to create them.
-

Medical device cyber security: 2022 Update includes review of regulations and implications in an ever-evolving landscape of vulnerabilities.
-

The FDA released a new Cybersecurity draft guidance on April 2022. It is intended to replace the current final guidance from 2014 which is well overdue an update. The draft guidance significantly expands requirements for cybersecurity activities and documentation for medical devices. The intention is to align medical device development with current best practices from other industries. This white paper reviews these new requirements and considers their impact on medical device developments.
-
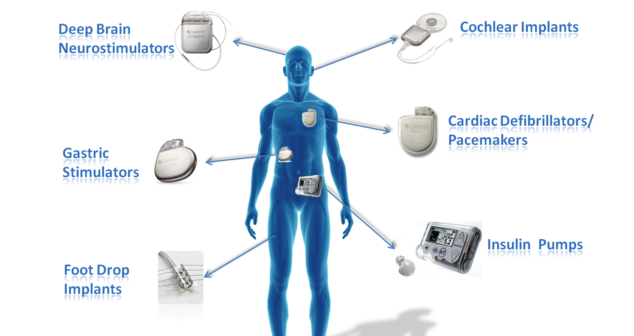
Basics of cyber security as it relates to medical devices, and five tips for developing hack resistant medical devices.