Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

2021 Update on Health Canada's medical device regulatory development during the COVID-19 pandemic and lessons learned from the experience.
-

Returning to Work Safely during COVID discusses documents and programs from Intertek, BSI and TÜV that assist ISO PAS 45005 compliance.
-

When COVID is Under Control – StarFish Medical employee choices for their first activities when COVID restrictions are eased.
-
Tips from StarFish manufacturing leaders on changes that we made for Manufacturing safety and efficiency during COVID-19.
-

Round up of Top 10 COVID-19 medical innovations from robotics to COVID 19 POC Diagnostic Tools, PPEs, and Portable dialysis machines.
-
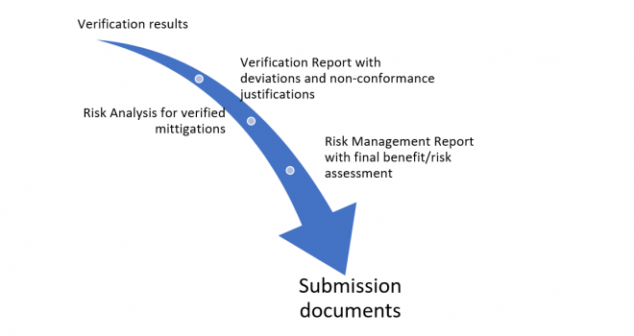
Author describes her experience submitting a medical device application (ventilator) under the Health Canada Interim Order.
-

Supply Chain constraints in the age of COVID-19 are troubling. Medical device purchasers supply chain tips for delivering the goods.
-
What is a coronavirus and why is a CoVID-19 Point of Care (POC) Diagnostic so important? Background and analysis of the vital role of POCs.
-

One response to possible COVID-19 medical device shortages is to try to hack DIY COVID-19 medical devices together. This is far more nuanced than it seems.