Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
The do's and don'ts of running Industry-Integrated Engineering Courses including University of Victoria's BME401C collaboration with StarFish.
-

Translating user and patient insight into tangible design offers tips and techniques for industrial designers and human factors researchers.
-
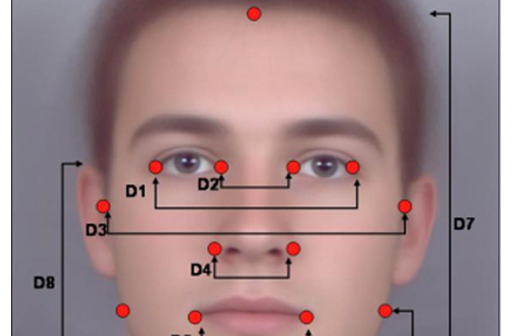
PM, EE, ME and ID/HF Manager insights and examples of how Medical Device Human Factors and ID impact Commercialization success.
-
Effect of Formative Test Fidelity shows the importance of formative testing and its impact on medical device design team usability data.
-

Formative Evaluations and Tests details the differences between these two methods, and the appropriate circumstances for their application.
-

Creative Human Factors Processes for COVID-19 solutions to combat logistical hurdles for human factors research and evaluation activities.
-

Alarm fatigue is influenced by a variety of factors. End user and patient behaviours are most identifiable through procedural observation.
-
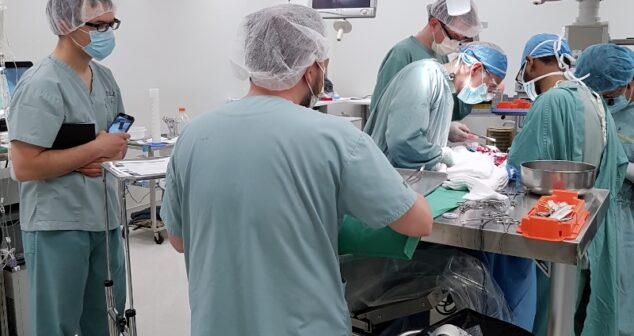
Procedural observations of medical procedures are invaluable and inform designs with perspective of users, procedural workflow and intended use environment.
-
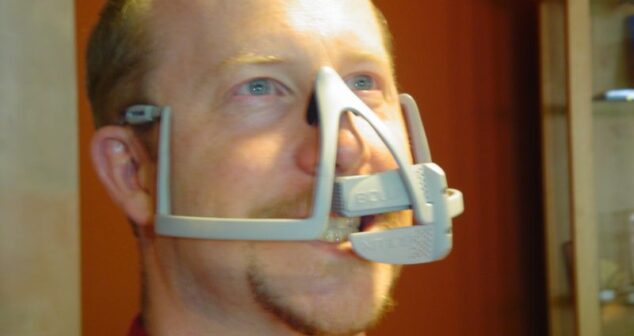
Exploration of applying IEC-62366-1:2015 or human factors engineering to the design of medical devices compared to the FDA guidance document.