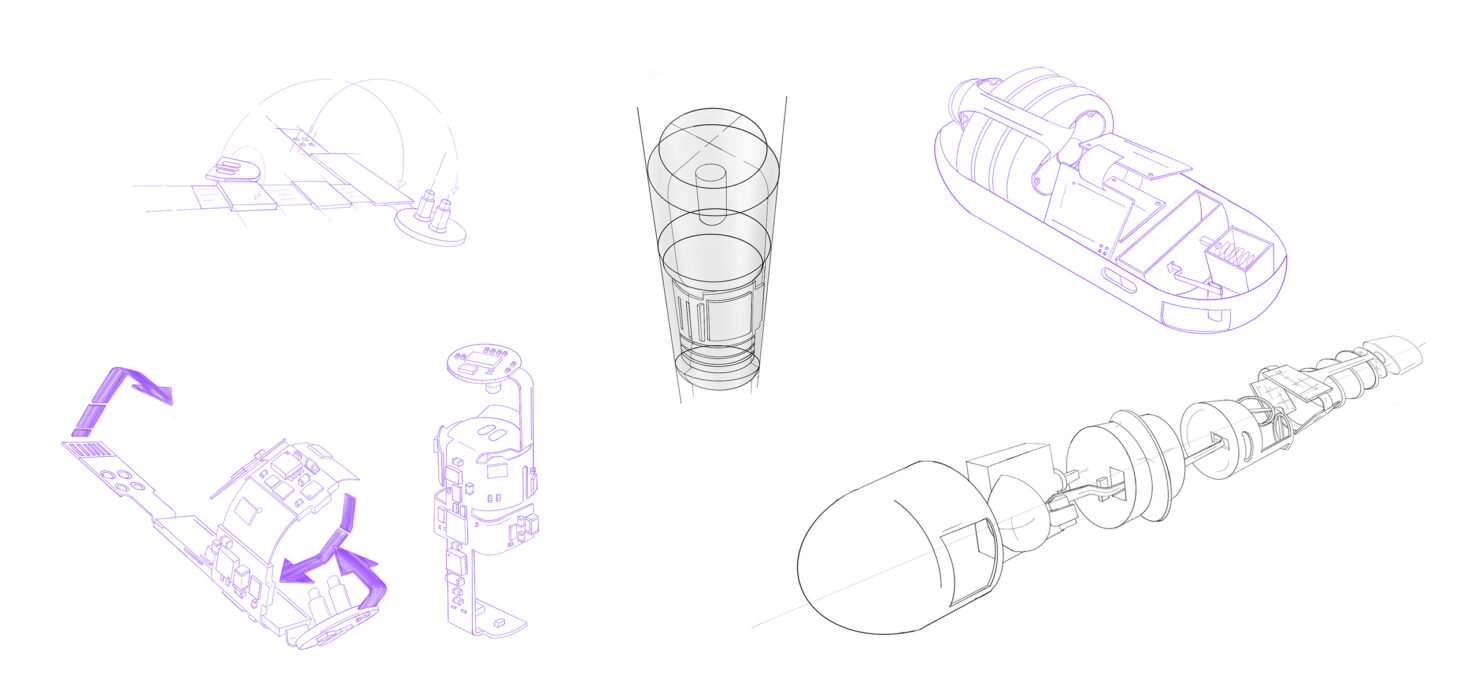
Medical Device Design & Development
Custom medical device design and development from concept to final product
You’ve got the vision. We help bring it to market.
Bringing a medical device to market is one of the hardest things you’ll ever do. Whether you’re advancing a breakthrough idea or driving the next wave of innovation inside an established company, the challenges are relentless. Technical hurdles stack up against shifting regulations, while every decision carries consequences for patients, investors, and your team.
Success demands more than clever engineering. It takes a medical device design and development partner who sees the whole landscape, untangles complex problems, and guides you with confidence from first concept to commercial launch. That’s the role StarFish plays, helping innovators turn bold ideas into market-shaping devices that improve lives and define industries.

Concept Development for Medical Devices
Turning vision into a viable medical device takes more than technical skill — it requires clear alignment around user needs, product-market fit, and regulatory expectations. We work closely with your team to define the product from every angle, ensuring that what we design is not only innovative, but feasible, fundable, and built for commercial success.
User- and Patient-Centric Medical Device Design
Getting this stage right can make or break your device’s success. At StarFish, we take a deeply user- and patient-centric approach — ensuring that every design decision is grounded in real-world needs, clinical workflows, and regulatory expectations.
Our team helps you move beyond concepts and assumptions to create precise, manufacturable designs that support safety, usability, and commercial viability. We bring the technical depth, cross-functional insight, and rigorous design controls to help you de-risk this critical stage — so you can advance with clarity and confidence.


Rapid Prototyping for Medical Device Development
Each prototype is a critical learning tool — not just a box to check. In MedTech, small oversights can become costly delays or regulatory barriers later. We help you move quickly and intelligently through iterations, combining rapid prototyping with rigorous testing and risk management.
Our goal is to help you uncover and resolve potential issues early, validate key assumptions, and refine usability — all while keeping development aligned with your regulatory path and market strategy.
Ready to bring your vision to life?
Verification, Validation, and Clinical Prototypes
Verification and validation is where your device must prove itself — to regulators, to clinicians, to investors. We understand how pivotal this stage is to your overall success.
Our team brings deep experience in developing clinical-grade prototypes and managing the complex documentation and testing required for submission and trial readiness. We help ensure your device not only meets regulatory standards, but performs reliably in the hands of real users and patients.


Regulatory Strategy for Medical Devices
Regulatory strategy isn’t just about compliance — it’s about enabling progress. Missteps can stall your timeline, burn investor trust, and derail commercial plans. That’s why we embed QA/RA expertise into every stage of development, not just at the end.
We work as an extension of your team to align design decisions, risk management, and documentation with your regulatory pathway — helping you move forward faster and with fewer surprises.
Technology Transfer and Manufacturing Scale-Up
Scaling from prototype to production is where many devices stumble. Maintaining design integrity, managing supplier complexity, and meeting regulatory requirements at volume is not trivial.
We guide you through this high-risk transition with a clear focus on quality, consistency, and risk reduction. Our expertise in technology transfer helps protect your investment and prepare your device for successful launch and sustainable production.


Medical Device Program Management
Complex MedTech programs involve many moving parts, stakeholders, and risks — and poor coordination can quickly derail progress. We provide dedicated, experienced project and program management to keep everything aligned — timelines, technical progress, regulatory strategy, and business goals.
Our approach helps ensure momentum, transparency, and accountability across the entire team — so you can drive the program forward with confidence.

Matt Mitcho
Founder, CEOGemelli Biotech“As we worked through the project, it was not only an engineering firm developing an instrument, but it was a consultancy, as well. That allowed us to do it properly to make sure that the CLIA-certified laboratory was happy with the LDT that was developed at the lab. We also envision these instruments going through 510(k) approval to be sold to external laboratories, academic institutions, or large GI practices, and we know that StarFish has the capabilities to help us through that journey.”

Erik Henne
Vice President of Research and DevelopmentUptake Medical“StarFish in one word? “Boutique”, that really means “Bespoke”. We are ourselves a boutique medical device company. We are very focused on single product platform. We are not a giant diversified company, but we have important things happening here and the size and scale of StarFish matches our size and scale.”
How We Support Your Medical Device Journey
No two medical device journeys are alike. We bring a flexible, collaborative process that adapts to your needs — whether you’re building first-of-kind technology, preparing for trials, or scaling an existing platform.
Our proven stage-gate framework, combined with deep MedTech expertise, helps you balance innovation with compliance — giving you the structure to manage risk and the flexibility to innovate at pace.
Why MedTech Innovators Choose StarFish
MedTech innovators partner with StarFish because we understand both the science and the business of medical devices. We bring the technical depth, regulatory insight, and practical experience to help you overcome complexity, accelerate progress, and deliver devices that improve lives.
We’re not just a design shop — we’re a strategic partner invested in your success.
TL;DR / Key Takeaways
- Bringing a novel medical device to market is complex — StarFish helps you navigate it with confidence.
- Proven expertise in medical device product design and development, from concept through clinical trials and launch.
- Deep user- and patient-centric approach ensures designs meet real-world needs and regulatory requirements.
- Integrated QA/RA strategy to align development with regulatory pathways and accelerate approvals.
- Trusted partner for scaling devices from prototype to full production with reduced risk.
Ready to advance your device?
Let’s talk about how we can help you navigate complexity and bring your innovation to market.
Explore Our Services
Related Resources

Electrocardiography (ECG) remains the gold standard for non-invasive cardiac assessment, providing a vital window into the heart’s electrical health. By recording electrical impulses through surface electrodes, clinicians can identify life-critical conditions such as arrhythmias, myocardial infarctions, and conduction abnormalities.
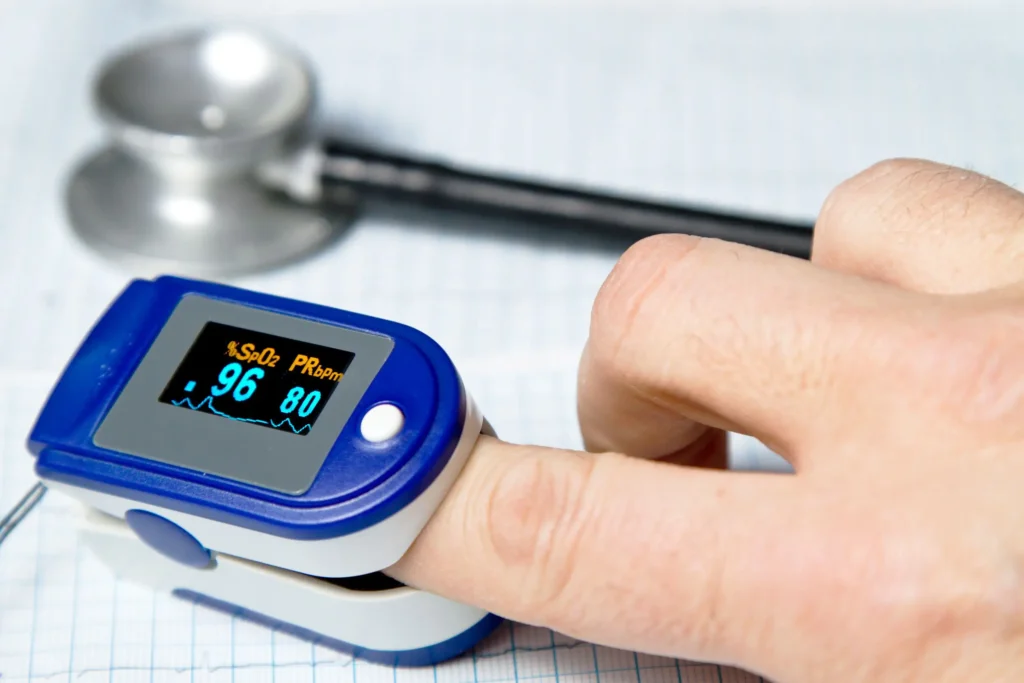
Wearable medical and wellness devices are increasingly commonplace in the healthcare field. For example, devices such as smartwatches incorporate sensors to provide continuous health tracking data to their users.

Nick and Nigel explore how much bacteria can exist on devices and why it matters. They explain that bacteria are everywhere.