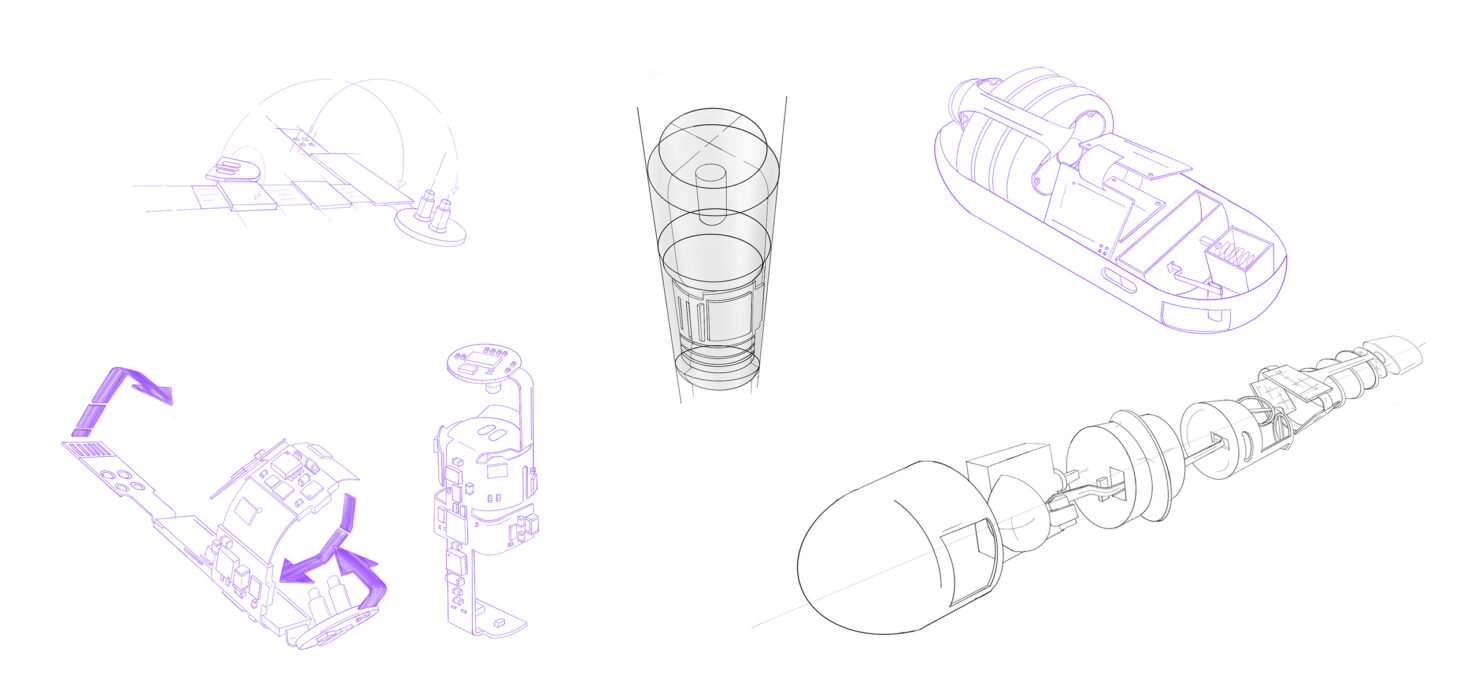
Product Design & Development
Custom product design and development from concept to final product
You’ve got the vision. We help you bring it to life.
StarFish Medical offers full product design and development engineering services that propel your concepts from initial idea to final market-ready product. With a multidisciplinary team of experts dedicated to your project’s success, you’re assured a final product that meets the highest standards of safety, efficacy, and user experience.

Concept Development and Product Definition
Our product design and development process starts with a deep dive into your vision, market needs, and regulatory requirements, ensuring we have a shared understanding of your overall objectives. Our team of engineers, scientists, and designers then collaborates with you to explore possibilities, hone concepts, and transform your product into a viable solution.
Early-stage product design and development activities include:
- User needs analysis
- Product conceptualization
- Feasibility studies
- Preliminary design sketches
Initial Product Design
With the concept identified and agreed upon, our engineers and designers take a user- and patient-centric approach to develop detailed designs and engineering plans. Leveraging advanced modeling and simulation tools, we bring your vision to life with precision and creativity.


Rapid Prototyping, Testing, and Iterating
At StarFish, we champion iterative development with a keen focus on risk management. By rapidly creating prototypes, we can test and refine your design early in the process, ensuring a faster time to market.
Our in-house prototyping capabilities enable us to swiftly produce and evaluate physical models. This thorough vetting of design iterations ensures that each stage is meticulously reviewed before advancing to the next phase.
Ready to bring your vision to life?
Building Prototypes for Verification & Validation and Clinical Trials
When you need a pilot prototype for verification and validation (V&V), pre-clinical, or clinical trials, StarFish Medical has the expertise and facilities to support you.
Our dedicated new product introduction (NPI) and manufacturing teams build prototypes in our class 10,000 cleanrooms (when required), ensuring seamless production without the need to transfer to another manufacturing partner. This streamlined approach minimizes transitions, reduces risk, and provides you with greater control over the initial product builds, bringing your innovative solutions to life with precision and efficiency.


Strategic Regulatory Support
Successfully bringing a medical device to market requires a clear regulatory roadmap and compliant systems, processes, and documentation. Our quality assurance and regulatory affairs (QA/RA) experts offer invaluable guidance throughout the development process. With decades of experience working with regulatory bodies worldwide, our QA/RA team assists with everything from regulatory strategy to submission package preparation, ensuring your product meets all necessary standards and requirements.
Manufacturing and Technology Transfer
Minimize manufacturing risks with a seamless transition from prototype to full-scale production. We collaborate closely with you and our manufacturing partners to ensure a smooth technology transfer. Throughout all design and development stages, we leverage project management best practices to uphold the highest quality standards and optimize production efficiency.


Project and Program Management
To enhance efficiency and accelerate time to market, every StarFish client is assigned dedicated project and program managers. Utilizing proven agile processes and a rigorous quality management system (QMS), we ensure your program stays on track and aligned with both internal and external milestones.

Matt Mitcho
Founder, CEOGemelli Biotech“As we worked through the project, it was not only an engineering firm developing an instrument, but it was a consultancy, as well. That allowed us to do it properly to make sure that the CLIA-certified laboratory was happy with the LDT that was developed at the lab. We also envision these instruments going through 510(k) approval to be sold to external laboratories, academic institutions, or large GI practices, and we know that StarFish has the capabilities to help us through that journey.”

Erik Henne
Vice President of Research and DevelopmentUptake Medical“StarFish in one word? “Boutique”, that really means “Bespoke”. We are ourselves a boutique medical device company. We are very focused on single product platform. We are not a giant diversified company, but we have important things happening here and the size and scale of StarFish matches our size and scale.”
Ready to bring your vision to life?
Our Process
At StarFish Medical, we follow a stage-gate process for developing novel medical products. Our multidisciplinary team of engineers collaborates closely to advance product design and development through each stage. At every stage gate, an expert team evaluates whether the design and program meet the necessary criteria. The documentation generated throughout our product design and development process aligns seamlessly with the content required for regulatory submissions.
Having worked with hundreds of clients, we understand that each project is unique. Our systems and processes are designed for flexibility, allowing us to adapt our approach to meet your specific needs—even if that means deviating from our standard product design and development methodology. We can join your journey at any point.
We can jump on board at any part of your journey.
Whether you have a nascent product idea that needs conceptualization or a mature working prototype that requires refinement, StarFish can partner with you at any stage of the product design and development journey. We tailor our approach and involvement to the project’s specific needs, ensuring we provide the right support at the right time.
We create maximum value.
We understand what it takes to move your vision forward. We also know what it takes to design, develop, test, and build all types of medical devices.
We thrive on technical challenges and often come up with novel, out-of-the-box solutions. Maximizing value by designing and supporting the right product is key to our success. That’s why we always start with a user-centric design approach to transform visionary ideas into cutting-edge medical products.
Explore Our Services
Related Resources
Ariana and Mark walk through FDA-approved options and explain how to select the right one for your product. From metals to plastics and electronics, not all devices can handle the same process.
In this episode of MedDevice by Design, Ariana and Mark dive into the biomechanics and materials science behind osseointegration for implants.
Nick and Nigel dive into the world of jet injector drug delivery. This needle-free method, made popular in science fiction and real-world vaccines, is still used today.