
Medical device design & development
Empowering MedTech innovation to create breakthrough products
that improve health and save lives
At StarFish Medical, we blend deep technical expertise, regulatory insight, and user-centered design to bring MedTech innovation to life. From startups to global enterprises, we turn visionary ideas into breakthrough products that improve health and save lives.
25+
Years
200+
Professionals
1000+
Programs
Our Services
Working with StarFish
From early-stage startups to global MedTech leaders, we help companies solve complex challenges and turn bold ideas into market-ready products.

Meet ELLA
Introducing ELLA, an AI-powered tool designed to transform how medical device companies navigate regulatory compliance.

Latest Resources

From how much of your body is actually bacterial to how fast microbes can multiply, these facts are designed to stick with you long after the party ends.
In medical device development, verification is both a safeguard and a stress test, not just for the product, but for the process.

In the world of medical device development, requirements are often treated as a regulatory tax, essentially documentation created solely to satisfy a compliance need.
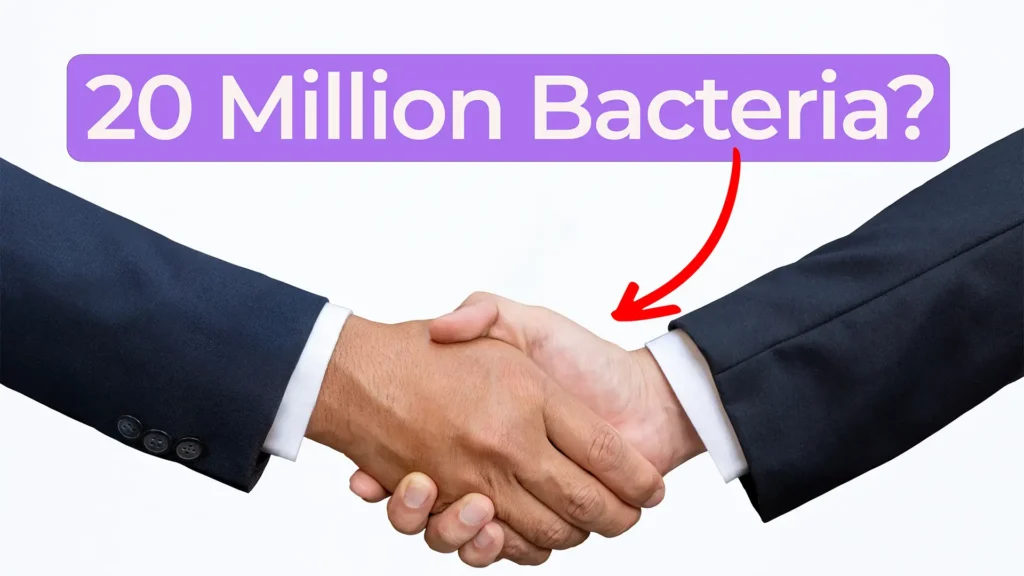
In this Bio Break episode, Nick and Nigel explore a surprising and memorable microbiology fact that puts everyday hand hygiene into perspective.
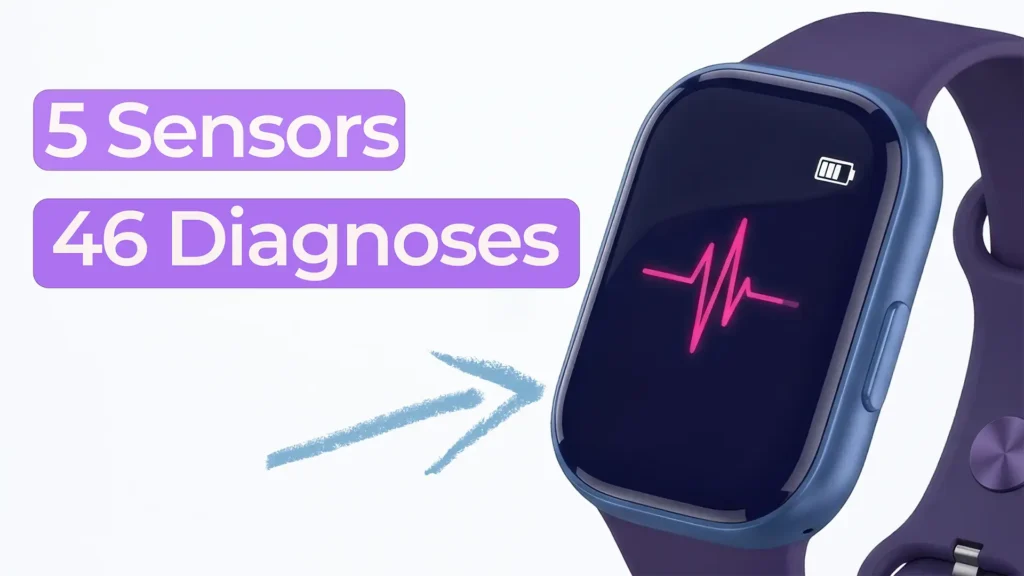
Nick and Nigel explore how a surprisingly small set of sensors could be used to identify a wide range of common health conditions.
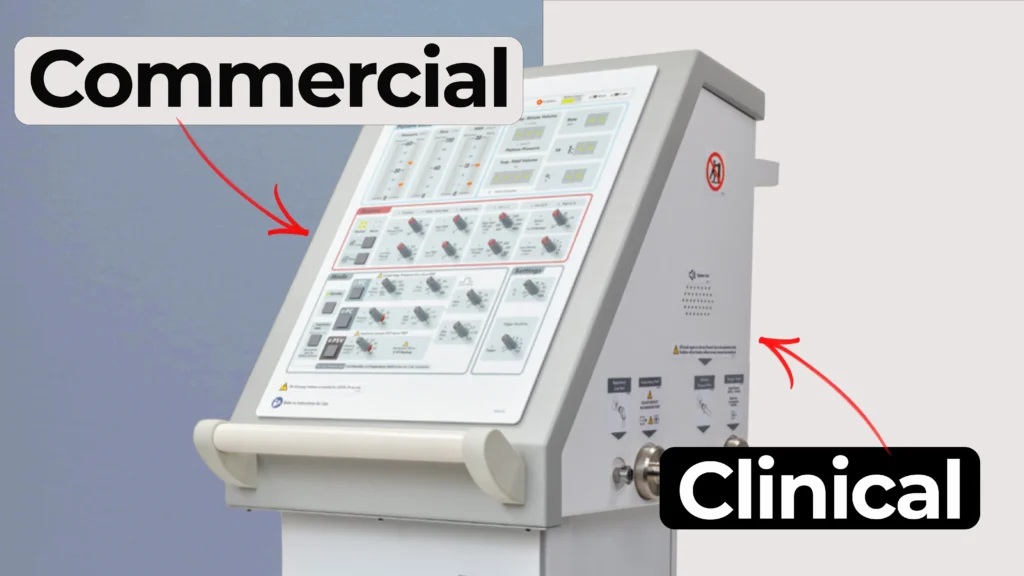
Understanding how clinical ventilator development differs from commercial ventilator design is essential for teams planning early studies.
Sectors We Serve
Axolotl BrainPrint Microsphere Generator

Aurora DNA Sample Preparation Device
Join over 6000 medical device professionals who receive our engineering, regulatory and commercialization insights and tips every month.