Cell and Gene Therapies (CGT) are revolutionizing modern medicine by offering potential cures for previously untreatable diseases. However, the effective delivery of these advanced therapies poses unique challenges. Herein, we explore five key challenges associated with CGT products that need to be considered for effective treatment and discuss how innovative Drug Delivery Devices for Cell and Gene Therapies can address these issues.
Challenge 1: The CGT product needs accurate delivery to a hard-to-reach location in the body.
Drugs are frequently taken orally or administered via injection for systemic treatment in the body. However, many CGT products must be delivered to a specific location in the body to realize their therapeutic benefit, and often, these locations in the body are hard-to-reach. For example, there are several CGT products that require delivery to the brain for the treatment of Parkinson’s disease and other neurodegenerative conditions, such as ALS, both areas that we have direct product development experience. In these instances, without adequate targeting of the cell or gene therapy, the treatment is not effective.
In many cases, effective drug delivery is enabled by delivery devices that are specifically designed, developed and tested with the therapeutics. Highly specialized systems that use microcatheters and advanced imaging-guided delivery systems, can facilitate the accurate placement of CGT products. These devices can navigate through the intricate pathways of the body to deliver therapeutics precisely where needed, make treatment less invasive and reduce systemic side effects.
Areas of the body that are frequently targeted for precise delivery include the brain, the heart, the spine, the pancreas, the eye or the ears. And although development of a drug delivery combination device adds costs to the development process and eventual patient therapy, these costs are justified by the unique (even curative) efficacy of many CGT products.

Challenge 2: CGT products are often administered as complex formulations.
Many CGT products are formulated in complex, often high-viscosity solutions, making them difficult to administer through conventional delivery systems. For example, hyaluronic acid (a naturally occurring polysaccharide) is often used in cell therapy formulations due to its favourable biocompatibility profile and cell suspension/dispersion properties. The viscosity of these types of formulations is highly dependent on the molecular weight and concentration of the hyaluronic acid used but is generally orders of magnitude higher than simpler water-based formulations.
Depending on the nature of the final formulation, off-the-shelf drug delivery systems may not be available for precise and accurate injection of the CGT product. Especially when a large volume of a viscous cell therapy product needs to be administered, a custom delivery solution may be required. Fortunately, a variety of novel drug delivery devices have been engineered with suitable fluid injection mechanisms capable of handling viscous formulations. Advanced wearable syringe pumps and auto-injectors with high-force delivery capabilities (e.g., the Ypsomed YpsoMate auto-injector) are examples of Drug Delivery Devices for Cell and Gene Therapies that can effectively administer these viscous solutions without compromising the integrity of the therapeutic agents or causing significant discomfort to the patient.
Join over 6000 medical device professionals who receive our engineering, regulatory and commercialization insights and tips every month.
Challenge 3: CGT products are sensitive to fluid flow conditions.
CGT products are composed of large, conformation-specific biomacromolecules and/or particles that are almost always sensitive to mechanical stress, such as fluid shearing forces. These forces can deform and degrade the CGT products by inducing changes to their native structures, reducing or altering their efficacy. That is why the fluid flow dynamics of any CGT delivery device must be well understood and controlled as a key parameter of the device design and development process.
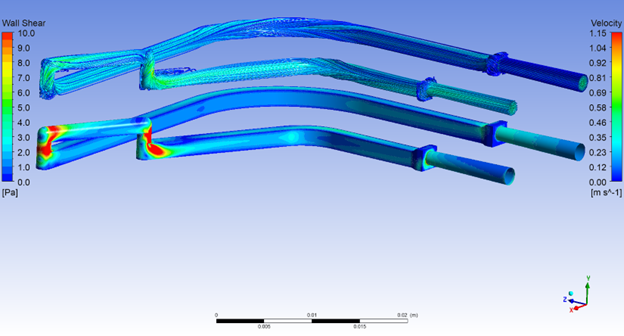
Mapping the fluid path of the delivery device and performing detailed fluid simulations can help address any potential problems in the prototype design. Using this approach, we have successfully eliminated areas of shear stress for several products that were suffering from abrupt fluid transitions, in-line turbidity or bubbles.
In general, minimizing transitions by using smooth, wide-bore needles and advanced pump systems (such as the Medfusion 4000) that regulate the flow rate to prevent rapid changes in pressure and velocity all help minimize unwanted fluid strain on vulnerable drug products. Finally, real life, hands-on testing to determine potency of the drug product before and after handling in the delivery device is essential to verify the efficacy of the delivery process in a suitable pre-clinical model.
Challenge 4: CGT products are often stored in cryoprotectants that must be removed post-thaw, before administration.
Typically, non-CGT drug products are stored in their final formulation before administration to the patient. This is purposeful because it minimizes processing of the drug at the patient’s bedside. However, CGT products often require cryoprotectants for long-term stability and viability during storage at -80°C. These cryoprotectants must be removed immediately before administration, necessitating aseptic processing—such as filtration or centrifugation—post-thaw.
Ideally, the preparation and administration of CGT products, including the removal of cryoprotectants, should occur outside the constraints of dedicated GMP clean rooms. Therefore, the entire workflow, from preparation to administration, must be carefully considered when designing an aseptic drug delivery system. Post-thaw processing of CGT products may also introduce additional testing requirements to ensure the safety and efficacy of the final product after cryoprotectant removal.
An optimal Drug Delivery Device for Cell and Gene Therapies should facilitate a robust and streamlined CGT preparation and administration process, enabling these critical steps to be performed safely and effectively at the patient’s bedside.
Challenge 5: CGT products need expensive infrastructure for drug administration.
In addition to needing expensive specialized, complex manufacturing processes, CGT products also often depend on highly specialized infrastructure for effective drug administration. This presents a significant challenge to scaling these therapies.
By developing a custom drug delivery device that simplifies the route of administration, CGT manufacturers can increase the accessibility of their products to a broader population. For instance, consider a CGT product that must be delivered to a specific area of the brain, which currently requires access to a small set of hospitals equipped with an intra-operative MRI surgical suite. By creating a delivery device that emphasizes usability and precision, and eliminates the need for intra-operative MRI, the CGT product can be implemented in a larger number of hospitals, making it accessible to many more patients worldwide.
Conclusion
The delivery of CGT products presents unique challenges that require innovative solutions. Custom Drug Delivery Devices for Cell and Gene Therapies play a crucial role in overcoming these obstacles, ensuring that these groundbreaking therapies reach their full potential. By addressing issues such as targeted delivery to hard-to-reach locations, handling of high-viscosity formulations, sensitivity to shearing, removal of cryoprotectants, and removing the need for complex infrastructure, these devices are critical in enabling the full potential of CGT products for patients.
As technology continues to evolve, we can expect further advancements that will enhance the delivery of CGT products, ultimately improving patient outcomes and transforming the landscape of modern medicine.
Gary Skarja is a Bio Services Program Manager at StarFish Medical. Over his 23-year career in the commercial life sciences sector, he has led the development of new medical materials & devices, regenerative medicine technologies and single-use biomanufacturing tools at companies ranging from pre-revenue start-ups to a multibillion dollar global organization. Gary holds a B.Eng. and M.Eng. in Chemical Engineering from McMaster University and a Ph.D. in Chemical Engineering from the University of Toronto.
Joris van der Heijden is Bio Services Program Manager at StarFish Medical. Previously he was Director of R&D at Spartan Bioscience where he led the development of a point-of-care COVID-19 diagnostic test. Joris received his PhD in infectious diseases from UBC.
Read a review of the FDA Guidance on Using ISO 10993-1 integrating biocompatibility evaluation within the risk management process, as outlined in the FDA Guidance Document Use of International Standard ISO 10993-1, Biological Evaluation of Medical Devices Part 1: Evaluation and testing within a risk management process.
