
Electrical Engineer’s Overview of IEC 60601-1 Scope and Normative References
Sorting through the alphabet soup of the many standards that apply during medical device development can be daunting for startups & founders. This blog gives an overview of one of the more important ones: IEC 60601-1. The first installment covers Scope and Normative References. Part 2 covers detailed descriptions of the sixteen sections.
StarFish Medical follows standards that regulate our Processes and others that regulate our Products. For example, our processes are driven by the requirements of ISO 13485 and the US FDA cGMP, while the products we design are regulated by FDA 510(k) and PMA requirements, IEC 62304, IEC 61010-1, and many others. One product standard we deal with frequently is IEC 60601-1 Medical Electrical Equipment – Part 1: General Requirements for Basic Safety and Essential Performance. Let’s distill its essence to help readers understand where some of our design decisions come from.
First a bit of background. The International Electrotechnical Commission (IEC) authors many standards within the electrotechnology field, however, since they are a non-governmental organization their standards do not have the force of law unless adopted by national regulatory bodies. The IEC 60601 series of standards has been adopted by the European Union, Canada, the USA and many other countries (sometimes with national deviations). Hence you may see references to CAN/CSA-C22.2 NO. 60601-1 (Canada), EN60601-1 (EU), UL60601-1 (USA) and similar, all referring to substantially the same requirements.
IEC 60601-1 is the parent of a series of standards relating to the safety of Medical Electrical Equipment. Underneath it there are:
- IEC 60601-1-xx Collateral Standards which specify additional specific requirements, e.g. 60601-1-2 covering EMC (Electromagnetic Compatibility)
- IEC 60601-2-xx Particular Standards which modify, replace or delete 60601-1 requirements for specific equipment, e.g. IEC 60601-2-37 for ultrasound diagnostic equipment.
IEC 60601-1 also cites Normative references. These are other standards whose requirements are included by reference. e.g. IEC 60417-DB Graphical symbols for use on equipment
Join over 6000 medical device professionals who receive our engineering, regulatory and commercialization insights and tips every month.
IEC 60601-1 Scope and Normative References – When to Use Them
How do we know when IEC 60601-1 is the correct standard to use? If your device has an electrical component, and has an Applied Part or transfers energy to/from the patient, and is intended to monitor, diagnose or treat a medical condition, then IEC 60601-1 very likely applies. Its section 1 (Scope) and section 3 (Terminology & Definitions) assist in confirming applicability. Sometimes other standards will apply instead, such as IEC 61010-1 which covers In Vitro Diagnostic equipment, or ISO 14708-1 for Active Implantable medical devices.
There is a common misconception, based on the title of the standard, that IEC 60601-1 covers only electrical safety. In fact, while it applies to Medical Electrical Equipment, the requirements cover a gamut of potential hazards. It is sometimes even used to document safety of devices that have no electrical component, when there are no other more appropriate standards. Here are its sections:
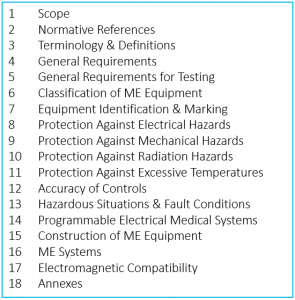
Throughout the text we find a useful convention that has been adopted by many standards – the use of the following terms:
May describes a permissible method of meeting a requirement
Shall marks a mandatory requirement
Should indicates compliance is recommended but not required
Bjarne Hansen, MESc. is a former StarFish Medical Senior Electrical and Biomedical Engineer. An avid sailor, he is also author of our most read blog for many years: Inexpensive prescreening strategies for medical device EMC compliance.
Images: StarFish Medical