
Addressing ingress protection for home healthcare medical devices
What the standard says and what you should know.
Increasingly medical device technology is filtering down from hospitals and into patients’ homes. Designers need to understand and address the impact of this transition on the medical device design when addressing ingress protection for home healthcare medical devices
60601-1-11 Collateral Standard: Requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment introduces additional IP requirements beyond those in 60601-1, that cover a larger classification of devices and may impact a home healthcare device. These requirements are intended to protect patients and devices against the increased likelihood of liquid ingress in a home use environment and the associated risks such as electrical shock or impaired functionality.
Ingress Protection (IPN1N2) covers both particulate and liquid ingress into an enclosure, where N1 defines the particulate requirements and N2 defines the ingress of liquid requirements. This blog will focus on protection against liquid ingress in a home use environment, which is often the more challenging of the two. For additional information, see Dana Trousil’s Ingress Protection blog, which focuses on Cleanability, Orientation, and Heat dissipation.
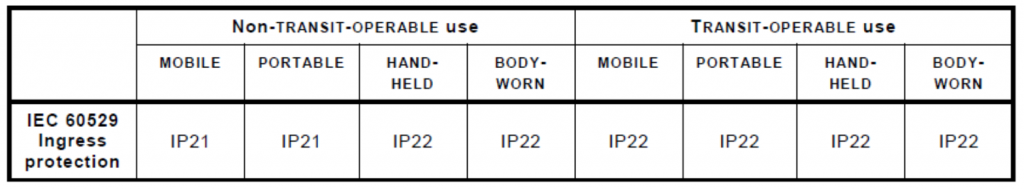
Additional requirements for IP ingress protection have been added through clause 8.3.1 of 60601-1-11. Noteworthy additions include the requirements that all “TRANSIT-OPERABLE, HAND-HELD, and BODY-WORN ME (Medical Electrical) EQUIPMENT shall maintain basic safety and essential performance for at least IP22… AND all other ME EQUIPMENT shall maintain basic safety and essential performance for at least IP21” (IEC60601-1-11 Collateral standard: systems used in a home healthcare environment).
This means that all devices intended for use in a home healthcare environment must have some level of ingress protection. The only exceptions to this being FIXED or STATIONARY ME EQUIPMENT which are not defined in the standard.
Also, for PORTABLE ME EQUIPMENT that are only intended to be used inside a carrying case, the IP requirements only need to be met by the carrying case. Figure 1 below outlines a summary of ME Equipment IP requirements based on use cases for the Home Healthcare environment.
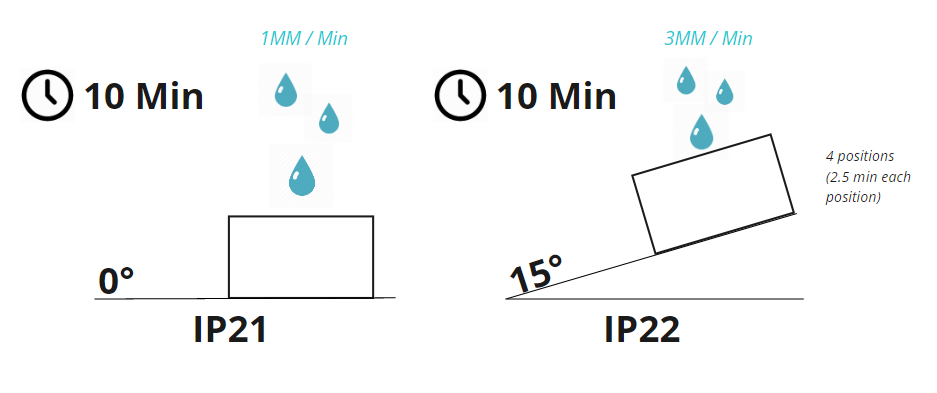
The methods for testing ingress protection are outlined in ISO 60529. In this standard, IP21 is defined as protection against vertically falling water at a rate of 1mm rainfall per minute for a total of 10 minutes while rotating the device on a turntable (1 RPM). IP22 is defined as protection against vertically falling water at a rate of 3mm rainfall per minute for a duration of 2.5 minutes in each of four fixed positions of tilt (10 minutes total / no turntable). The figure below illustrates the two testing conditions.
In addition, 60601-1-11 states that the device should be tested while in the least favorable position of NORMAL USE. “Least favorable” means the position resulting in the highest risk of fluid ingress and resulting risk to basic safety and essential performance, while “NORMAL USE” is defined as “operation, including routine inspection and adjustments by an operator, and stand-by, according to the instructions for use”. This means that normal use extends beyond when the device is simply being used by a patient, and includes situations such as storage, stand-by use, maintenance, transport, and inspection that a user may need to do. Identifying the least favorable position of NORMAL USE can be especially tricky for hand-held or body worn devices. The least favorable position of normal use can be identified by studying the device’s workflow, use cases, usability, and human factors.
For example, when identifying the least favorable position of a body worn device, consider the situations when the device is being worn, charging, stored, or simply laying around the home. It may be that the device has a different exposed surface while being worn compared to when the device is placed on a nightstand or table. What is the level of risk to basic safety and essential performance in each of these positions if the device is accidentally exposed to liquids? As the device’s design continues to evolve, the least favorable position could be subject to change and should be re-evaluated on a regular basis.
Understanding all the possible positions of normal use is also important when defining proper handling instruction and storage methods in the instructions for use (IFU). Although providing instructions, warnings, and suggestions in the IFUs is a great idea, these may not be suitable as mitigations since users are not always guaranteed to follow the instructions. As such, IP mitigation strategies will still need to be included to protect users against the risks associated with fluid ingress. These risks may include electrically shorting the electronics or shocking your patient.
IP requirements for a medical device can have significant impacts to the device’s design and development timeline if they are not properly assessed early on. The risks to essential performance and patient safety due to fluid ingress should be discussed and evaluated prior to architecture definition and enclosure design. Once the risks are understood, the methods and design strategies to mitigate them can be explored, if mitigations are needed at all.
Design–based mitigation strategies addressing ingress protection for home healthcare medical devices may take the form of gaskets, adhesives, geometry changes, and meshes. The designer should also consider the impacts of these IP strategies on other parts of the system (cables, ventilation, user controls) and how they might negatively affect other design requirements such as mechanical stress, thermal management, manufacturing viability and product lifetime validation.
One of the best strategies when brainstorming how to design ingress protected enclosures is to study other consumer products that have an IP rating. Some good examples include waterproof bike lights, car key fobs and water proof headphones.
Following up with a robust testing strategy is crucial to ensure IP mitigations are effective. Using rapid prototyping parts and materials to expedite testing do not always provide an accurate representation of how the final material / process will perform during IP testing. Solely relying on prototyped parts may result in false positives or false negatives due to the differences in tolerances, surface textures, wicking properties and contact angles between materials and manufacturing processes.
Addressing ingress protection for home healthcare medical devices requires ingress protection for the majority of medical device types because it is reasonable to foresee that the devices may accidentally be subjected to liquids resulting in risk of harm to the patient. A thorough understanding of applicable portions of 60601-1-11 and 60529 standards, along with a robust risk management strategy, can help drive informed design decisions early on in development and a better chance of successful IP conformance.
Joshua Coutts is at Mechanical Engineer at StarFish Medical. He received the Chris Denny Memorial Award for Innovation for his work developing a new generation of nasal drug delivery system. Josh is a graduate of the University of Victoria and a very active member of our Victoria social committee.
Photo credit: Googooms blood pressure monitor image – AliExpress
