Why Microneedles Still Matter in Drug Delivery
In this episode of Bio Break, Nigel and Nick explore microneedle drug delivery—a growing field in medtech that aims to improve patient comfort and treatment compliance. While the term “microneedles” may sound futuristic, this technology has been around for years. The goal? Deliver medicine through the skin with less pain, less fear, and fewer barriers to care.
What Is Microneedle Drug Delivery?
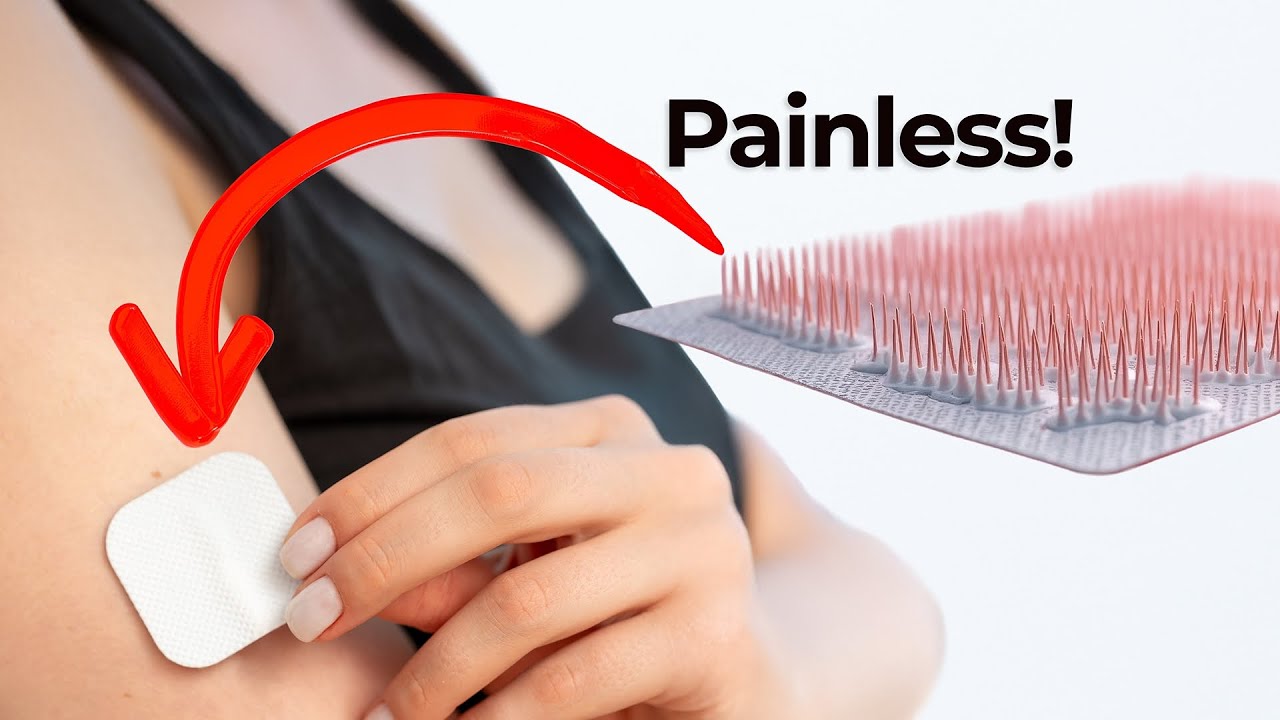
Microneedles are tiny structures, often as small as a human hair, that can puncture the skin’s outer layer to administer medication. Some dissolve into the skin, while others act as miniature hollow syringes. Unlike traditional injections, microneedle drug delivery can avoid deeper nerve endings—meaning less pain for the patient.
Advantages Over Traditional Injections
The key benefit of microneedles is improved patient compliance. Many people skip essential treatments due to needle anxiety. By minimizing discomfort, microneedles make it easier for patients to stick with treatment plans. This approach could be especially useful for vaccines, hormone therapies, or chronic care where regular administration is needed.
Limitations and Drug Compatibility
Not all medications are suitable for microneedle delivery. Larger molecules may not pass through the tiny channels or could be damaged by shear forces. Product developers must consider drug formulation, molecule size, and delivery speed when selecting this method.
Future Outlook and Applications
Microneedle drug delivery has potential in both consumer health and clinical use, but widespread adoption depends on finding scalable applications. The technology continues to evolve, particularly in pairing with biologics and nanoparticles.
Related Resources


Nick and Nigel walk through how sterile disposables are processed and verified before they reach the field.

The FDA agentic AI is making headlines after the agency announced its own internal AI review tool. In this episode of MedDevice by Design, Ariana and Mark discuss what this could mean for medical device submissions and regulatory efficiency.

The sandwich ELISA assay is one of the most common ELISA formats used in diagnostics. Nick and Nigel walk through the method step by step using simple visuals and plain language.