
Bio Break: Thanksgiving Traditions and Lessons for Medical Devices
In this episode of Bio Break, StarFish Medical experts Joris van der Heijden and Nick Allan celebrate Thanksgiving by sharing a heartwarming and hilarious family tradition: preparing the perfect turkey. Through this tale of generations working together to create the iconic holiday meal, they uncover a surprising lesson that applies to medical device development and procedural design.
Nick recounts a story from his family’s Thanksgiving tradition, where four generations worked together to prepare a turkey, following a long-passed-down method involving a pot of water to keep the turkey moist. However, this year’s preparation revealed a surprising twist—the tradition originated not from culinary precision but from a practical fix for an old, unsteady stove! This discovery sparks an insightful discussion about the importance of understanding why each step in a process exists, whether in cooking or designing medical devices.
The story transitions into a valuable takeaway: in medical device development, blindly following procedures without questioning their purpose can lead to inefficiencies or errors. Much like uncovering the reason behind the family’s turkey tradition, developers must critically evaluate instructions for use (IFUs) and procedures to ensure each step serves a meaningful purpose.
Key insights from the episode include:
- Traditions and Processes: The importance of understanding the origins and reasoning behind longstanding methods.
- Critical Thinking: Always question procedural steps to identify and remove outdated or unnecessary instructions.
- Practical Application: Applying these lessons to medical device development ensures efficiency, precision, and safety in design and operation.
This Bio Break episode offers a lighthearted yet meaningful look at how family traditions can teach us about innovation and critical thinking. It’s perfect for medical device developers, healthcare innovators, and anyone who loves combining life lessons with professional insights.
Thanksgiving Traditions and Lessons for Medical Devices
Related Resources

A structured, well-documented design review process is a critical component of successful product development, particularly in the medical device industry.

In medical device development, we deal with complex projects that span multiple disciplines, timelines, and regulatory gates. It’s a constant balance between moving fast enough to innovate, but slow enough to stay compliant.
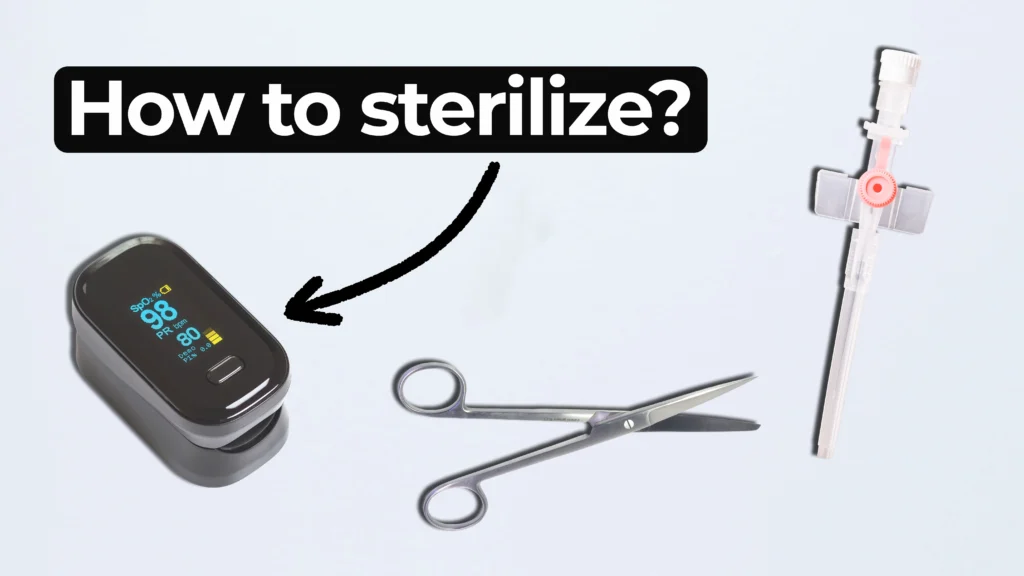
Ariana and Mark walk through FDA-approved options and explain how to select the right one for your product. From metals to plastics and electronics, not all devices can handle the same process.
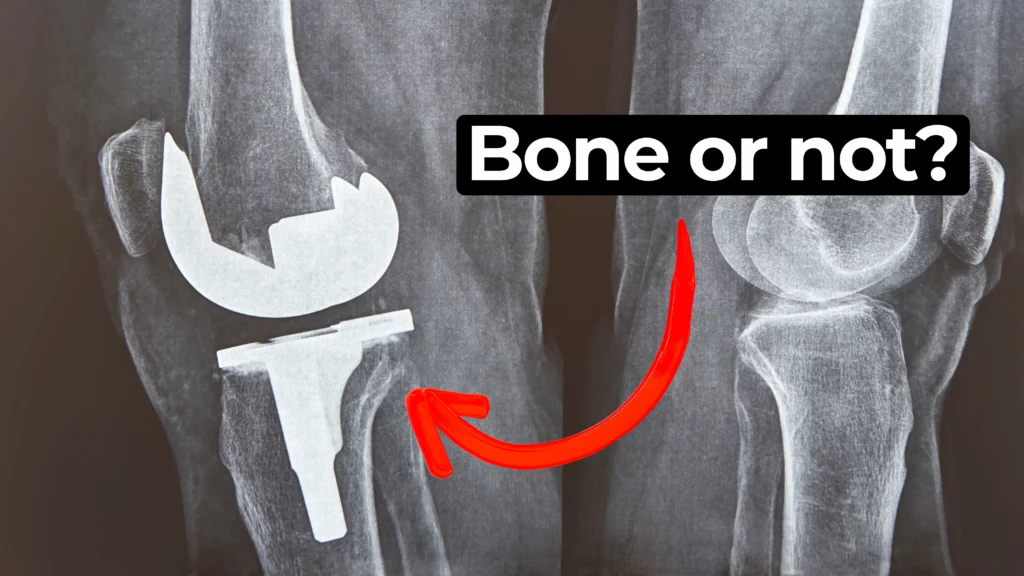
In this episode of MedDevice by Design, Ariana and Mark dive into the biomechanics and materials science behind osseointegration for implants.
Learn how StarFish Medical led a consortium that created a Ventilator 2.0 therapy device in record time.