
Bio Break: The Power of Continuous Analyte Monitoring
In this episode of Bio Break, StarFish Medical experts Joris and Nick dive into the transformative concept of Continuous Analyte Monitoring (CxM) and its growing role in wearable medical devices. They discuss the value of tracking metabolic markers over time, as opposed to relying on static time-point measurements, and how this approach enhances precision in both diagnostics and treatment.
The discussion highlights the evolution of continuous monitoring, starting with the gold standard of blood testing. While blood samples provide accurate results, their invasive nature and impracticality for frequent testing have driven innovation in non-invasive or minimally invasive techniques. For instance, wearable devices now use sensors with flexible needles or interstitial fluid measurements to monitor markers like glucose continuously.
Nick shares fascinating insights from his past work monitoring stress responses in animals, where cortisol levels were measured using both blood samples and innovative hair analysis techniques. He emphasizes the importance of timing in traditional testing, as data can fluctuate significantly depending on the time of day or external stressors. This variability underlines the immense value of continuous monitoring, which provides consistent, real-time data and eliminates the need for rigid sampling schedules.
Key takeaways from the episode include:
- Continuous Monitoring Advantages: The ability to track trends and patterns over time, offering deeper insights into a patient’s health.
- Wearable Innovations: How cutting-edge devices measure biomarkers like glucose or cortisol through interstitial fluid, enhancing patient convenience and compliance.
- Precision in Data: Continuous tracking minimizes variability caused by time-of-day effects or environmental stressors, improving diagnostic and therapeutic accuracy.
This episode highlights how CxM technology is shaping the future of personalized healthcare by offering real-time, actionable insights. Whether you’re a developer, healthcare professional, or simply curious about wearable medical devices, this episode provides valuable knowledge about the impact of continuous monitoring on patient outcomes.
The Power of Continuous Analyte Monitoring
Related Resources
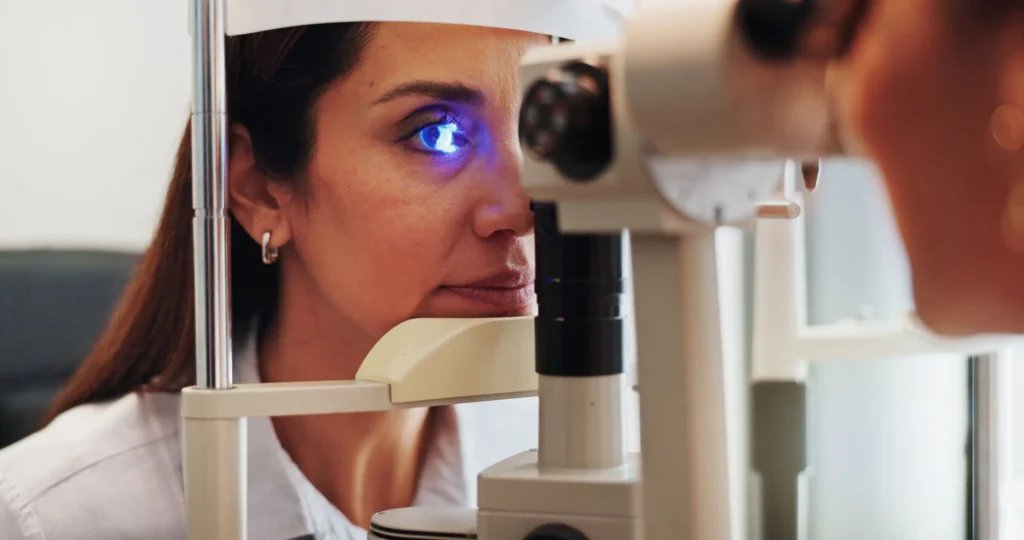
I was recently looking through the OPTICA trade journal Optics and Photonics News – specifically its summary of “Optics in 2025.” A few highlights were of particular interest to me in terms of their potential applicability to future medical devices.
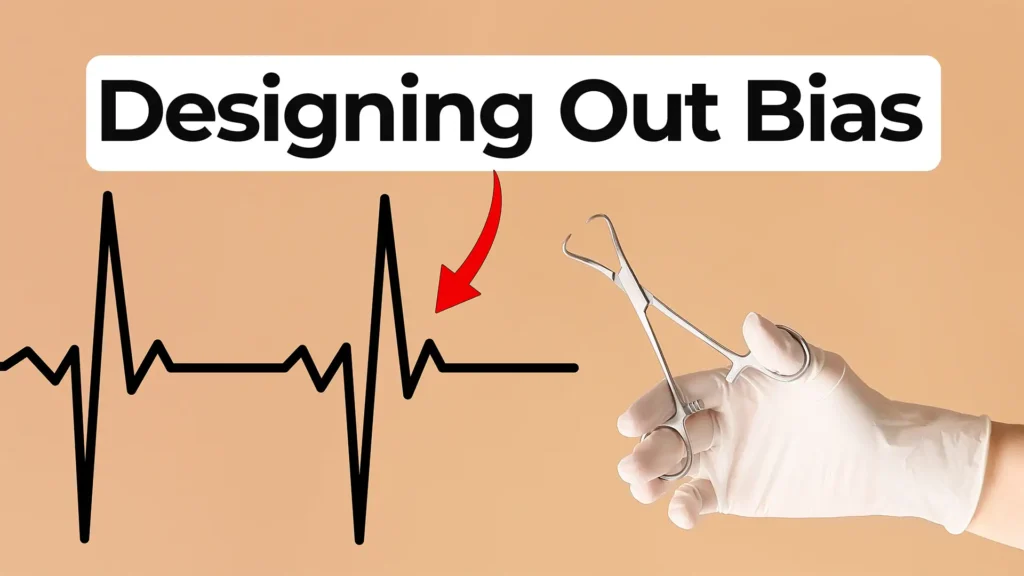
Ariana Wilson and Mark Drlik explore how bias can enter the development process and why engineers and manufacturers must actively work to prevent it.

Did you know that 5-8% of total national carbon footprints come from the healthcare sector? Much of this (around 80%) is general waste – such as from office work – and the rest (~20%) requires special handling due to its dangerous nature.

Electrocardiography (ECG) remains the gold standard for non-invasive cardiac assessment, providing a vital window into the heart’s electrical health. By recording electrical impulses through surface electrodes, clinicians can identify life-critical conditions such as arrhythmias, myocardial infarctions, and conduction abnormalities.
Learn the Basics of Wearable Medical Device Adhesives including DO’s and Don’ts in our comprehensive overview.
Related Resources

I was recently looking through the OPTICA trade journal Optics and Photonics News – specifically its summary of “Optics in 2025.” A few highlights were of particular interest to me in terms of their potential applicability to future medical devices.

Ariana Wilson and Mark Drlik explore how bias can enter the development process and why engineers and manufacturers must actively work to prevent it.

Did you know that 5-8% of total national carbon footprints come from the healthcare sector? Much of this (around 80%) is general waste – such as from office work – and the rest (~20%) requires special handling due to its dangerous nature.

Electrocardiography (ECG) remains the gold standard for non-invasive cardiac assessment, providing a vital window into the heart’s electrical health. By recording electrical impulses through surface electrodes, clinicians can identify life-critical conditions such as arrhythmias, myocardial infarctions, and conduction abnormalities.
