Clean or Sterile medical device: How clean does it need to be?
Medical devices often come into contact with patients, and users. Sometimes repeatedly. Addressing patient and user contact is a critical aspect of medical device development. Biocompatibility is a significant aspect of contact, but what about sterility? And if a device does not need to be sterile, then how clean does it need to be?
How Clean or Sterile Should my Medical Device Be?
The level of cleaning and disinfection needed depends on the use of the device. All reusable devices need to be able to be cleaned to some level, and that level depends on the risk of the device. In 1957, E.H Spaulding devised a method of classification that has been in use since. Although today’s knowledge of microorganisms is stretching the limits of this classification, it remains as the basis of classification today. Many hospitals and health boards have a list of recommended practices which outline the requirements for reusable equipment.
The classification first aligns the criticality of the device. The criticality of the device is dependent on the contact: does the device comes in contact with mucous membranes, or sterile cavities, or only skin contact.
Based on the initial classification, a level of cleaning and disinfection is advised. The classifications are as follows:
Non-critical
Non-critical items are those that have skin contact only, or do not enter sterile body cavities or mucous membranes. Examples are stethoscopes, blood pressure cuffs, and patient lift slings.
Cleaning with low-level disinfection is advised, depending on the likelihood of contamination by pathogens. In some cases, only cleaning may be warranted. The cleaning protocol will need to be cleared by the FDA as part of any 510K submission.
Semi-critical
Semi-critical devices contact mucous membranes, but do not contact sterile cavities. Examples are laryngoscopes, respiratory therapy equipment, anesthesia equipment, sonography equipment, CPR face masks, etc. Cleaning with sterilization is advised, with high level disinfection if sterilization is not feasible.
Critical
Critically classed items are those that come into contact with sterile bodies, implantables, and so forth. Examples are surgical equipment, forceps, biopsy equipment, eye equipment including soft contact lenses, athroscopes, laparoscopes, and broncoscopes. Sterilization is required.
The following table illustrates the classifications:
| Classification | Definition | Level of Processing | Examples |
| Critical | Equipment that enters sterile tissue, including the vascular system | Cleaning following by Sterilization | Surgical Instruments Implants Biopsy Instruments Foot Care equipment Eye and Dental equipment |
| Semi-critical | Equipment that comes into contact with non-intact skin or mucous membrances (but does not penetrate) | Cleaning following by high-level disinfection Sterilization is preferred | Respiratory equipment Anesthesia equipment |
| Non-critical | Skin contact or non-contact devices | Cleaning followed by low-level disinfection, or via cleaning alone | ECGs Oximeters Bedpans, commodes, etc. |
Reusable versus Disposable
Cleaning reusable equipment is a process that needs to be validated. The FDA released a guidance on reprocessing, updated in 2017.
Disposable equipment follows the same requirements as reusable – the Spaulding classifications govern the cleanliness required. The risk of contaminants through the manufacturing process is generally small, but not zero, and so the risks for pathogens needs to be addressed through the risk assessment process.
Critical devices must be sterile, and semi-critical needs to be taken on an individual basis. Bioburden and bacteria count also depend on the type of bacteria present, which is why it’s important to look at risks associated in each case and develop disinfection protocols for those risks.
Unfortunately, there is no easy answer when it comes to cleaning and sterilization. Each device will need to be assessed based on the risks involved. The best course is to engage with a professional firm to determine what protocols are required, and what validation will be required. It’s also important to ensure that the devices meet any functional requirements after validation to ensure the device has not been damaged by the sterilization or cleaning process and continues to be safe and effective.
Sterilizing and Cleaning Background
Now that I’ve answered the question, “How clean does a medical device need to be”, it’s important to understand important sterilizing and cleaning terms and regulations.
Bioburden
Bioburden is defined as the amount of microorganisms living on the surface of a part or a component. Normally, bioburden is measured/defined before any sterilization, and is used mostly in the context of microbial limit testing for quality control during manufacturing.
The typical household surface has between 103 and 105 bacteria on it, depending on how frequently it’s handled, and the location of where the surface is, e.g. hand rail, kitchen. If you are interested, here is some research on how clean the average surface is. Bioburden testing is governed through ISO 11137 and 21 CFR part 211.
Cleaning agents
There are numerous cleaning agents, but not all are created equal. Germicides are agents that disinfect germs, as opposed to sporicides (spores), or fungicides (fungus), and so forth. All antiseptics are germicides, but not all germicides are antiseptics. Antiseptics are germicides specifically designed for skin or tissue contact. Enzymatic cleaners can be focused on a particular target. Lipases target fats, amylases act on starches, and proteases act on proteins, for example. Types of cleaners and their effects on microorganisms are extensively researched.
Join over 6000 medical device professionals who receive our engineering, regulatory and commercialization insights and tips every month.
Types of Pathogens
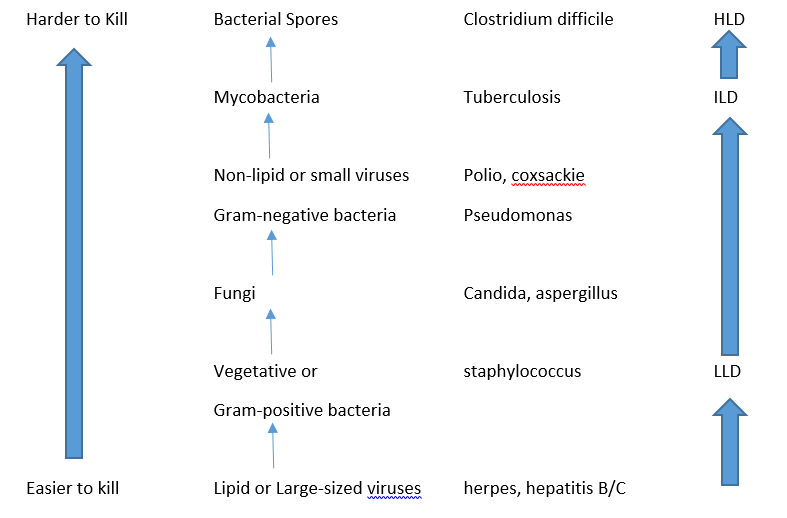
Bacteria has many classifications, but one method is to look at the peptidoglycan layer of the bacteria. Peptidoglycan is a layer of sugars and amino acids surrounding the bacteria. Gram-positive bacteria (named after Hans Christian Gram, Danish bacteriologist who developed the test) have a thick outer shell of peptidoglycan. Gram-negative bacteria have a thin peptidoglycan layer that is internal to the cell wall. The reason why the Gram test is important in disinfection is that the thick peptidoglycan wall in Gram-positive bacteria absorbs toxins and materials more readily (hence it absorbs the dye in the Gram test). Gram-positive bacteria, such as Streptococcus, are generally easier to kill as a result. So disinfection techniques that work on Gram-positive bacteria, may not work on the multilayered walls of the Gram-negative bacteria. Disinfection cleaners need to work on both types.
Some bacteria produce spores that are tough and more resistant to active cleaning. Endospores are a dormant form of the bacteria rather than a true spore, and can reactivate if the conditions are right. In general, endospores are produced by some forms of Gram-positive bacteria such as Bacillus or Clostridium. They are highly resistant to many actions of disinfection including UV, desiccation, extreme temperatures, and chemical disinfection.
Some classes of bacteria can form exospores, or microbial cysts. These are a dormant state of the organism where the metabolic rates of the bacteria is greatly slowed and the cell ceases activity. While resistant to disinfection, exospores are less resistant than endospores.
Mycobacteria is another serious family of bacteria, e.g. tuberculosis, which is highly resistance to disinfection techniques. The group grows in a way which resembles mold, hence the term “myco” meaning fungus. Disinfection techniques and efficacies are important when dealing with these bacteria.
Other pathogens include viruses, or fungi. Viruses can be classified loosely by whether they are enveloped in a lipid layer or not. Lipid viruses tend to be easier to kill, as are viruses that are larger, such as hepatitis B or C. Smaller and non-lipid viruses tend to have more resistance to disinfection, so viruses like Polio have higher resistances.
Cleaning
Cleaning is the act of removing soils and organic material from objects. Cleaning is not the same as disinfection. In fact, to have an effective disinfection method, cleaning is required. Soils and other organic material can shield microorganisms and prevent disinfection. Particular care must be taken where fluids have dried on to the surface, as cleaning can be more difficult.
Cleaning can be done a number of ways, but it’s usually mechanical and/or chemical in nature. Mechanical cleaning requires both a cleaning fluid, and a mechanical action. The action can be friction, such as rubbing or brushing, or some other method such as ultrasonic cleaners. The fluid is required to wash away areas where debris has been loosened by the mechanical means, or to reach those areas where mechanical means were not sufficient.
Chemical cleaning often uses near neutral or neutral detergents with enzymes added. Targeted cleaning solutions can break down specific proteins, fats, or starches depending on the targeted debris. It’s important to make sure that the base material is compatible with the cleaning solution and doesn’t adversely affect the material properties.
Disinfection and Sterlization
Disinfection, on the other hand, is the destruction of microorganisms on a surface. Here, a distinction between disinfection and sterilization must be made. Disinfection does not inactivate all forms of microorganisms. Depending on the level of disinfection, it may leave significant pathogens on the surface of the device. Because disinfection does leave microorganisms on the surface, disinfection can be broken down into several categories.
Disinfection Levels
- Low-level disinfection (LLD) is a process capable of killing most vegetative bacteria, some viruses, and some fungi. Note: This class of disinfection cannot be relied on to kill microorganisms such as mycobacteria, including Mycobacterium tuberculosis, or bacterial spores.
- Intermediate-level disinfection (ILD) is a process capable of killing vegetative bacteria, mycobacteria including Mycobacterium tuberculosis, fungi, lipid and nonlipid viruses. This class of disinfection will not necessarily kill bacterial spores
- High–level disinfection (HLD) is a process capable of killing vegetative bacteria, mycobacteria including Mycobacterium tuberculosis, fungi, and lipid and nonlipid viruses, and most but not all spores. Note: High-level disinfection is considered to be the minimum level of decontamination required for semi-critical medical devices.
Table of Antimicrobial Efficacy4
Sterilization
Sterilization, on the other hand, aims to kill all of the microorganism on the surface of a device. The sterility assurance level, SAL, is the odds of a microorganism existing on a surface after sterilization has been performed. So a 1 in a 1,000,000 chance would be expressed as a SAL of 10-6. A SAL of 10-6 or better is recommended for devices that penetrate normally sterile tissue, breached skin, implanted devices, etc. SAL’s of 10-3 are used for lower requirement systems, such as blood culture tubes or drainage bags.
There are a number of methods for sterilization, and each of these has their advantages and effectiveness.
Steam Sterilization
As the name indicates, steam sterilization uses high temperatures and water vapour to kill microorganisms. Autoclaves are steam sterilizers, and typically have four parameters: temperature, time, pressure, and steam. Steam is generally dry, saturated steam, meaning that water is boiled, then any remaining water in the vapour is vapourized further (>=97%).
Temperatures are typically 121°C (250°F), or 132°C (270°F), maintained for a set length of time, depending on the pressures used. Vacuum sterilizers evacuate the chamber of air, including from any pockets or pores within the device undergoing decontamination. Steam is then introduced, with the effect that the steam contacts surfaces more effectively. As a result, the sterilization times are greatly reduced, on the order of 5 minutes, versus 45 minutes for standard autoclaves.
Dry Heat Sterilization
Because of the lack of heat present in the water, dry heat sterilizers need temperatures significantly higher, 180°C (356°F), with longer times. However, if water can damage the components, then dry heat may be an option.
Ethylene Oxide (EO or EtO)
EO gas is typically used in initial sterilization due to the ability to sterilize large quantities at one time. EO reacts with DNA, proteins, or enzymes to disrupt cell activity. EO is toxic, however, and is highly reactive at low temperatures, which is another reason why EO is used in industrial settings.
Radiation Sterilzation
Gamma or electron beam sterilization is also an effective means of sterilization, and can be done through the medical device packaging. Dosing is important, and many plastics are not radiation stable (embrittlement or colour changes), meaning care must be chosen when designing devices where radiation will be used.
Whether clean or sterile applies, I hope the above information helps you determine how clean does your medical device (including therapy devices) need to be and how to address that need.
References
- ANSI/AAMI ST67:2019 Sterilization of health care products – Requirements for products labeled “STERILE”
- ANSI/AAMI/ISO 11137:2025. Sterilization of health care products
- https://www.fda.gov/media/80265/download
- https://cmr.asm.org/content/12/1/147; Antiseptics and Disinfectants: Activity, Action, and Resistance, Gerald McDonnell, A. Denver Russell. DOI: 10.1128/CMR.12.1.147
Dana Trousil is a StarFish Medical Mechanical Engineer and NPI Team Lead. He has successfully launched many products, with experience in a variety of processes, including NPI for medical devices.
Image: ID 98395851 © Flydragonfly | Dreamstime.com