
Regulatory Clearance through Computational Simulation: Industry Opinion, FDA Strategy, & Successful Case Studies
TL;DR
- Computational modeling (CM) is transforming medical device development, helping companies cut costs, risks, and time-to-market.
- FDA guidance on computational modeling, aligned with ASME V&V 40, now defines best practices for regulatory submissions.
- In silico clinical trials and virtual patient modeling can reduce reliance on bench testing and animal studies.
- Case studies show how finite element analysis (FEA) and computational fluid dynamics (CFD) accelerate device approvals.
- CM provides a proven pathway to regulatory clearance with validated digital evidence, driving faster, safer medical innovation.
Introduction
Computational Modeling and Simulation (CM&S) for medical devices has become a pivotal tool across the medical device industry, complementing and often enhancing traditional bench testing and clinical studies. Modern medical devices in cardiovascular, orthopedic, diagnostic, surgical, and many other categories increasingly rely on physics-based simulations. These include:
- Structural mechanics through FEA (Finite Element Analysis)
- Fluid flow through CFD (Computational Fluid Dynamics)
- Thermal effects on structures and fluids
- Electromagnetic effects on structures and fluids, and
- Multiphysics including some or all the above
These Computational Modeling (CM) simulations guide design decisions and predict device performance [1] [2]. The breadth of applications is evidenced by dozens of case studies – spanning orthopedics, cardiology, drug delivery, and more – that demonstrate how CM&S benefits medical device design across the board [2]. Each of these cases has been submitted to regulatory bodies – such as the FDA – with approval of the CM&S evidence for safety and efficacy. Cardiovascular stents and heart valves have extensively relied on simulation data, being evaluated for blood flow patterns and structural integrity under pulsatile pressures. Orthopedic implants – like hip and knee replacements or novel stemless shoulder implants – also undergo finite element stress analyses to optimize their geometry [3]. Additionally, implantable pumps or drug delivery devices can be tested in “virtual patients” to assess safety under various anatomical conditions [4].
In the early design phase, CM&S helps uncover design limitations and optimize device parameters before any prototype is built, reducing time to refine designs [1]. During verification, more mature development benefits from simulations by supplementing bench tests, exploring conditions that may be impractical to test physically. These include extreme physiological scenarios, large patient variability, non-discrete field studies, or inaccessible test areas [1]. Models are also very effective in evaluating parametric design spaces, such as assessing risks of stress and strain from varied orientation in stereotactic equipment, system thermal effects on critical enclosure fatigue, or drop analysis of PCBAs with shock-sensitive components.
In summary, CM&S can expedite all device development categories, supporting the total product life cycle from initial concept through post-market. As an approach to verifying a device’s safety and efficacy, CM&S provides a virtual testing environment that supplements and, in some cases, replaces bench and clinical testing. This enhances overall regulatory strategy and progresses devices through their submissions process [1].
Key Advantages of CM&S vs. Traditional Physical Testing
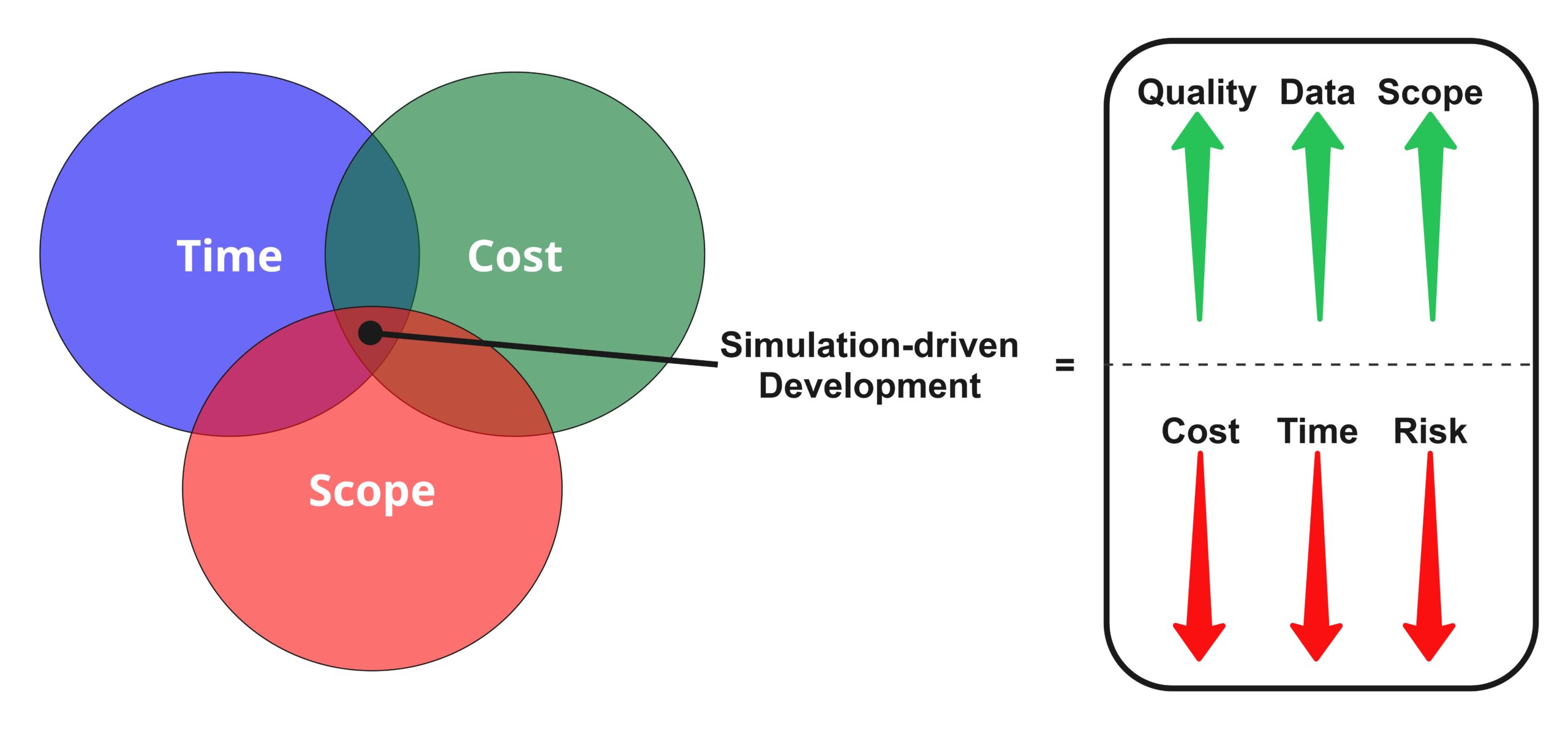
Simulation-driven medical device development can increase quality of outputs, data for decision making, and expand scope, all while driving down costs, timelines, and risks related to approach or the device itself. As a versatile approach, CM&S offers advantages over traditional methods of assessment like physical testing, including:
- Cost Effectiveness: Virtual testing can greatly reduce the number of physical prototypes and experiments needed, yielding substantial cost savings. Simulations allow developers to “fail fast” on a computer rather than in expensive lab or animal tests. Industry analyses have shown that CM&S can reduce product development costs while improving quality by streamlining testing through modeling. A recent industry report affirmed that organizations are saving millions of dollars and avoiding costly design reiterations late in development [5].
- Time Efficiency: CM&S accelerates time-to-market by shortening design-build-test cycles. Simulations run much faster than building and executing physical tests, enabling rapid iteration. Developers can quickly explore design variations or, with foresight, test multiple scenarios in parallel (e.g. various patient anatomies or usage conditions) without waiting for prototype fabrication. According to an MDIC industry survey, 69% of respondents strongly agreed that modeling and simulation helps reduce the time to market for their product [2]. In some cases, leveraging CM&S and a sound regulatory strategy has shaved up to 23 months off development timelines [5]. This compression of development time also enables the following benefits:
- a. Speeds patient access to innovations
- b. Extends the effective patent life of novel technologies
- c. Retains market exclusivity period for the manufacturer
- Safety and Risk Reduction: Using virtual models to evaluate device performance mitigates risk to both human and animal test subjects. Potential failure modes or hazardous scenarios can be investigated in silico without endangering patients. For example, the FDA has explicitly promoted CM&S clinical trials for medical devices – testing a device on a cohort of simulated virtual patients – to supplement or even replace certain clinical trials [6]. A landmark instance was the UVA/Padova human body simulator for insulin therapy devices, which the FDA accepted in 2008 as a complete replacement for preclinical animal trials in testing an artificial pancreas system [7]. By allowing developers to explore worst-case conditions (extreme anatomies, off-label use, rare complications) virtually, CM&S can identify problems early and improve patient safety. This approach also reduces ethical concerns and regulatory burdens by limiting animal experimentation and human trial exposure only to necessary cases. Overall, simulation provides a safe testing environment for iterative design refinement and failure mode analysis before any device is used in a living subject.
- Richness of Data and Insights: Computational simulations yield a depth and breadth of data that is often impractical or impossible to obtain from physical tests. While a bench test or animal study might provide a few outcome measures (e.g. pass/fail, pressure values at one location, etc.), a well-designed simulation can output detailed spatial and temporal information – for example: the stress distribution throughout an implant; blood flow properties (e.g., velocities and shear accumulation) in every region of a device; or temperature gradients during a thermal ablation treatment. This rich data output leads to deeper insight into how a device functions. Analysis engineers and project teams can probe the “why” behind device behavior by examining the effect of internal model variables. Moreover, CM&S enables extensive parameter sweeps or sensitivity analyses; dozens or hundreds of virtual experiments can map out performance across a wide design space or patient population. Regulators have noted that by pre-computing a large set of in vivo conditions, simulations can rapidly provide statistical and worst-case information about device performance without exhaustive physical testing [8]. Such comprehensive in silico datasets help engineers optimize designs with confidence and can reveal subtle interactions (for example, how slight anatomical differences influence an implant’s stability) that traditional testing might miss. In many instances, the insight gained from simulation can result in more robust, better-understood devices than with patient or animal model testing.
In summary, effective use of CM&S across all device classes cuts time, risk, and cost from development, while providing data richness and a higher quality device.
Opinions from Industry Experts: MDIC’s Landscape Report on CM&S
The Medical Device Innovation Consortium (MDIC) brought together directors and senior fellows from Medtronic, Boston Scientific, and Dassault Systèmes to author a landscape report, referenced throughout this blog. The paper highlights an industry survey of 41 medical device development professionals, the majority with engineering backgrounds. Most of the respondents worked at large established companies with >$1B revenue, with the second significant group comprising companies with revenues of <$1B. Select figures and context from the paper are shown in this section to illustrate key findings.

On opinions for areas for CM&S use, Figure 2 shows that those polled expressed high value in its use for Product Development/Testing and New Product Discovery, followed closely by Root Cause Analysis, Manufacturing, and Clinical Trial Development. This shows broad agreement on the versatility of CM&S across development stages.

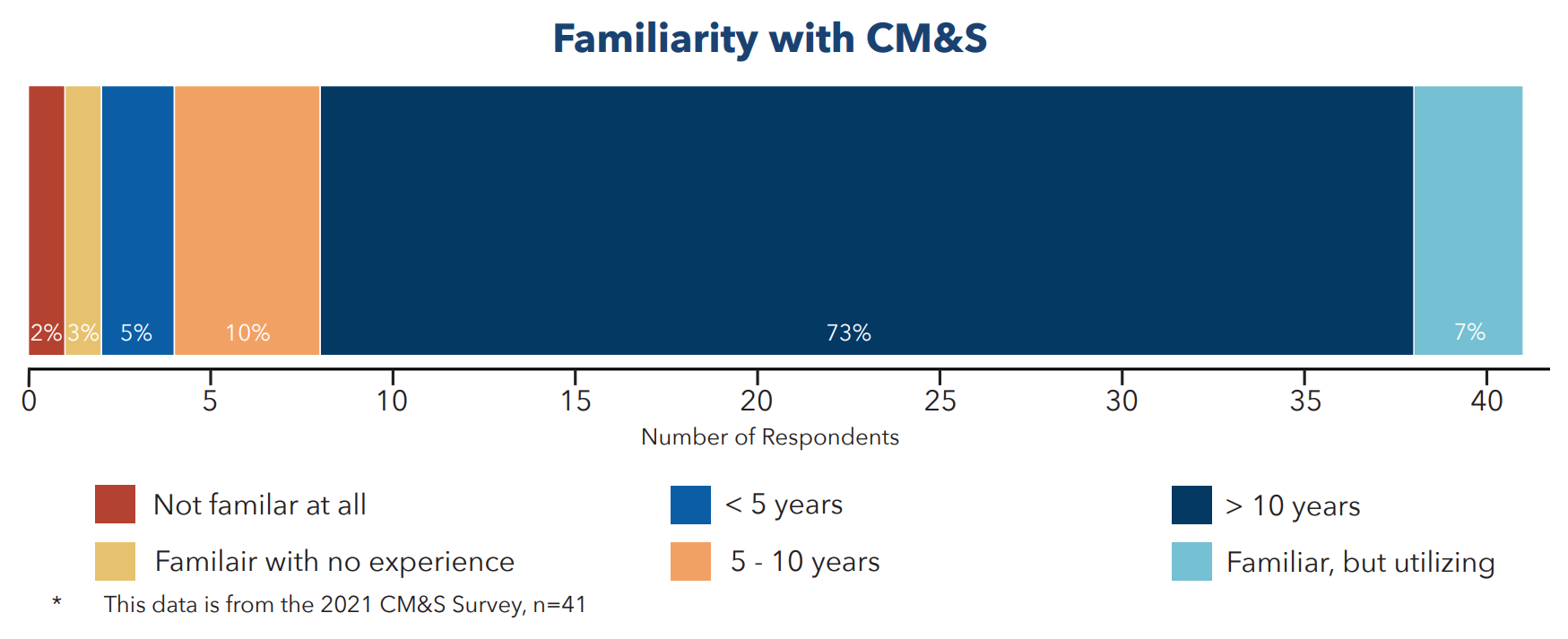
Noting a question on future applications for CM&S (Figure 3), respondents saw huge use cases for CM&S in replacing bench testing, animal/clinical testing, and expanding indications for use. There was little conjecture here, as 83% of respondents had over a decade of experience using these tools to develop products and deliver them to market (Figure 4).
Finally, across domains of interaction with CM&S, most respondents found Product Development was their main CM&S interaction (Figure 5).

All of this builds a compelling case that confidence is high for CM&S to make large impacts in med device product development in the years to come. But even if developers see the opportunities, how are regulatory agencies reacting to these novel approaches? It turns out they’re ahead of the curve.
FDA Guidance on Computational Modeling & CM&S Evidence in Submissions
Regulatory bodies worldwide, including the U.S. Food and Drug Administration (FDA), actively promote and recognize the value of CM&S as part of the evidence package for device approval or clearance. The past decade has seen an increase in manufacturers that use simulation results in their submissions to the FDA to support claims of device safety and effectiveness, in some cases using CM&S to supplement or replace physical/animal testing and clinical trials [6]. Early uses of CM&S in submissions were often ad hoc, but with the publishing of standards and regulatory guidances, a more structured approach for CM&S has arrived.
One clear indicator of this shift is the FDA’s recent guidance “Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions” (issued November 2023). This guidance is closely aligned with the ASME V&V 40-2018 standard and provides a structured framework for the risk-informed credibility assessment of computational models used in regulatory submissions [9]. Through this the FDA outlines how sponsors should validate and present their simulations so that regulators can trust the results. The guidance focuses on physics-based, mechanistic models (excluding purely AI or ML models) and defines model credibility as the “trust in the predictive capability of the model” for its context of use [10]. For a prescriptive 9-step framework to the process, see my colleague Dhruvitha Krishna’s blog on the topic. Otherwise, I offer some additional insights from the standard below.
Key elements of the FDA’s CM&S credibility framework (V&V 40)
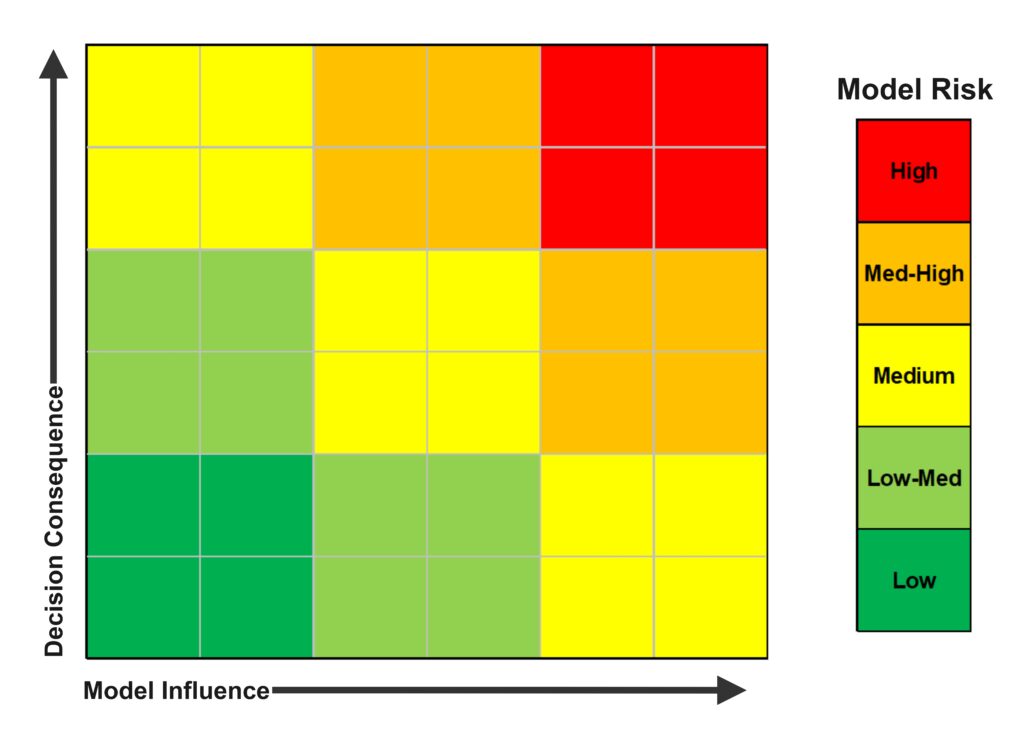
The approach is context-of-use driven and risk-based. Sponsors are expected to clearly define the “Question of Interest” that the model is intended to answer and the model’s “Context of Use (COU)” – essentially, how and for what decision the simulation will be used in the device’s evaluation. Based on the context, a “Model Risk Assessment” is performed to gauge the impact of a potential modeling error on the predicted medical device’s use. This approach considers factors like the significance of the model’s output to addressing device safety/effectiveness, especially around consequences if the model were wrong [10]. For example, a highly influential model that informs a high-risk decision (e.g., determining a life-critical device’s safety margin) would be labeled with high “model risk”, necessitating stronger evidence of credibility. Figure 6 highlights the gradient of increasing risk visually.
To build credibility, the framework outlines how Verification and Validation (V&V) evidence can be gathered in a structured way. The FDA guidance (mirroring ASME V&V 40) describes multiple categories of evidence across code verification, calculation verification, and validation [5]. At a high level, these assessment activities are:
- Code Verification: ensuring the software/hardware correctly solves the equations
- Calculation Verification: solving the model with sufficient accuracy, compiling potential errors in discretization (mesh), numerical solvers, and user inputs/outputs.
- Validation: activities comparing model predictions to physical data
One important note is these are not to be confused with device-level V&V of a device; rather, the guidance encourages sponsors to compile a body of evidence demonstrating that the model is solving the right equations correctly (verification) and predicting real-world behavior (validation), all within an acceptable range of accuracy for the intended context and risk. Validation evidence can span bench-top experiments, in vivo animal data, clinical data, population-based comparisons, analytical solutions, and checks for model plausibility and emergent behaviors [10]. This structure helps both industry and regulators systematically evaluate whether a simulation is reliable enough to factor into regulatory decisions [9].
Practically, the FDA advises sponsors to document their CM&S credibility process in a Credibility Assessment Plan and Report [5]. In many cases, engaging with the FDA early through the Q-Submission/pre-submission process allows modeling approaches to be assessed. The guidance recommends using these pre-sub opportunities to get FDA feedback on the proposed model risk categorization and planned V&V activities. When the time comes for formal submission (whether a 510(k), PMA, IDE, etc.), sponsors provide a Credibility Assessment Report summarizing the context of use, the model risk, the credibility goals, and all evidence showing those goals were met [5]. These steps are intended to increase FDA reviewers’ confidence in CM&S results and contextualize the evidence, making reviews more consistent and efficient as they were involved in the approach planning [10].
Notably, alignment with V&V 40 means that computational evidence is held to similar rigor as physical testing, streamlining communication of evidence that includes bench/clinical test data and simulation data. As one industry expert notes, this risk-informed framework aligns requirements for inclusion of physical and computational evidence, making it more convenient to use both in submissions and easing regulatory acceptance of modeling results [9].
The push for credible modeling is also aimed at addressing a known barrier: historically, many companies were uncertain about regulators’ expectations for CM&S [2]. In the 2021 MDIC survey cited before, 61% of respondents note “uncertainty in regulatory expectations” as the greatest barrier to wider adoption of modeling. With the V&V 40 guidance, the FDA has clarified those expectations by providing a clear methodology to demonstrate model credibility. This is expected to encourage broader use of CM&S in submissions, as companies can now follow an established blueprint to justify their models. To summarize, regulatory strategy has evolved such that a well-validated simulation, presented under the V&V 40 framework, can be a powerful piece of evidence in a medical device submission – potentially reducing the scope of required bench testing or clinical trials when and where appropriate. The FDA’s recognition of ASME V&V 40 and issuance of the 2023 guidance signal that CM&S is now a mainstream part of regulatory submissions for devices [5].
Case Studies: Successful Use of CM&S in Regulatory Submissions
Medtronic – Endovascular Devices: Medtronic leveraged finite element analysis (FEA) to simulate the deployment and mechanical fatigue of their Endurant II/IIs AAA stent graft system in complex aortic anatomies. CM&S helped evaluate long-term durability under physiological loading conditions. The FDA accepted this data to supplement bench testing and support the premarket approval (PMA) process. [11]

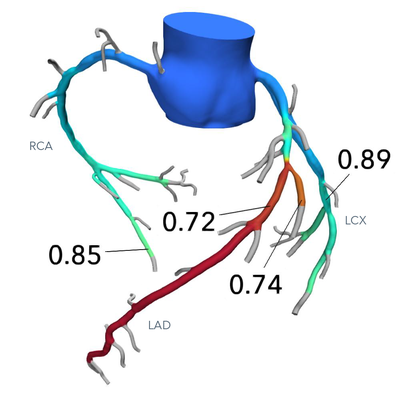
HeartFlow – FFRct Diagnostic Software: In their software, HeartFlow uses computational fluid dynamics (CFD) to create personalized coronary blood flow simulations from CT angiograms. This modeling approach replaces invasive fractional flow reserve (FFR) measurements. The FDA approved HeartFlow FFRct in 2014, making it the first non-invasive diagnostic software using CM&S for coronary artery disease. It is now a widely cited example in FDA digital health innovation. [13]
Smith & Nephew Variable-Angle Locking Mini-Fragment Plating System and EVOS Plating System: Smith and Nephew sought and were given two 510k’s, awarded in 2013 & 2014. These were for two plating systems intended for internal fixation of small bones and fragments (i.e., fracture fixation, arthrodesis, reconstruction…). These submissions included modelling via Finite element analysis (FEA) on all bone plates to predict the worst-case for subsequent non-clinical bench (mechanical) testing, allowing ~30 configurations to be assessed ahead of expensive and destructive physical tests.

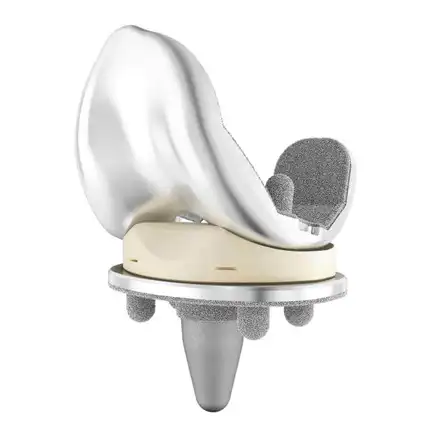
DePuy Graduated Compartmental Knee (GCK): A portion of the design underwent Finite Element Analysis (FEA) to support the claim of substantial equivalence. According to the 510(k) submission: “Finite Element Analysis was performed to demonstrate the substantial equivalence of the modified GCK unicompartmental femoral components to the predicate Sigma Unicompartmental femoral components and other clinically successful designs.” Two predicate devices were referenced in the 510(k), though neither explicitly described computational modeling as part of their justification. In modifying its previously cleared product to enable an extended flexion range, DePuy employed FEA as supporting evidence of safety and effectiveness to demonstrate substantial equivalence to the predicates. Note that DePuy is the orthopaedics division of Johnson & Johnson.
These case studies underscore how CM&S, when executed and validated rigorously, can directly contribute to regulatory approvals across multiple device domains. They also illustrate the complementary pairing of good modeling practices and regulatory requirements: in each example, credibility of the simulations had to be demonstrated (implicitly following V&V principles) to satisfy regulators. As more companies follow the FDA’s V&V 40 framework, the number of success stories is expected to grow, further cementing CM&S into the mainstream of medical device development and evaluation.
Conclusion
Computational modeling and simulation has evolved from a niche engineering aid into a critical enabler of medical device innovation. Across cardiovascular, orthopedic, diagnostic, surgical, and broader device categories, CM&S provides developers with powerful capabilities to design better products faster and more cost-effectively than ever before. By reducing reliance on trial-and-error prototyping and focusing testing resources where they are truly needed, simulation-driven development yields devices that reach patients sooner while enhancing confidence around safety and efficacy. The rich insights gained from CM&S lead to a deeper understanding of device behavior, which in turn drives smarter design iterations and more robust performance. Perhaps most importantly, the integration of CM&S into the regulatory process – epitomized by the FDA’s adoption of the V&V 40 credibility framework – has unlocked new avenues to use in silico evidence as part of official approval dossiers [9]. This not only accelerates development but also pushes the industry toward a future where virtual trials and digital evidence are standard practice alongside physical testing.
The strategic advantages of CM&S are clear. Compared to physical tests alone, they can provide:
- Lower development costs
- Shorter timelines
- Reduced risk
- A wealth of actionable data
With regulatory bodies now providing guidance on how to harness these advantages credibly, medical device companies have a blueprint to follow. Early adopters have already demonstrated improvements in product quality and performance through CM&S [2]. As frameworks like FDA’s V&V 40 become widely implemented, both industry and patients stand to benefit from the increased efficiency and innovation.
In summary, CM&S is not just a theoretical exercise but a proven, transformative approach to medical device development – one that, when validated and applied judiciously, offers a win-win of faster innovation cycles, robust assurance of safety and efficacy, and accelerated regulatory clearance through computational simulation for cutting-edge medical technologies.
References
[1] T. M. Morrison, M. L. Dreher, S. Nagaraja, L. M. Angelone and W. Kainz, “The Role of Computational Modeling and Simulation in the Total Product Life Cycle of Peripheral Vascular Devices,” J. Med. Devices, p. 5, June 2017.
[2] MedTech Intelligence, “Medical Device Computational Modeling and Simulation Reports Highlights Best Uses and Barriers to Adoption,” MedTech Intelligence, 14 Feb 2023. [Online]. Available: https://medtechintelligence.com/news_article/medical-device-computational-modeling-and-simulation-reports-highlights-best-uses-and-barriers-to-adoption/#:~:text=Case%20studies%20in%20the%20report,and%20simulation%20in%20product%20development. [Accessed 15 Apr 2025].
[3] D. Flynn, M. Palmer, R. Schiestl, S. Levine and T. Maeder, “Landscape Report & Industry Survey on the Use of Computational Modeling & Simulation in Medical Device Development,” [Online]. Available: https://mdic.org/wp-content/uploads/2023/01/CMS_Landscape_Report.pdf. [Accessed 15 Apr 2025].
[4] T. M. Morrison, “Virtual Patients for Regulatory Decision Making,” 18 September 2015. [Online]. Available: https://www.fda.gov/files/science%20%26%20research/published/Using-Modeling-and-Simulation-for-Medical-Device-Innovation–Virtual-Patients-for-Regulatory-Decision-Making-by-Tina-M.-Morrison.pdf. [Accessed 16 May 2025].
[5] NAMSA, “FDA Announces Release of “FDA Guidance: Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions”,” [Online]. Available: https://namsa.com/resources/blog/fda-announces-release-of-fda-guidance-assessing-the-credibility-of-computational-modeling-and-simulation-in-medical-device-submissions/#:~:text=,2%20of%20the%20draft%20guidance. [Accessed 15 April 2025].
[6] FDA, “Credibility of Computational Models Program: Research on Computational Models and Simulation Associated with Medical Devices,” 16 Nov 2023. [Online]. Available: https://www.fda.gov/medical-devices/medical-device-regulatory-science-research-programs-conducted-osel/credibility-computational-models-program-research-computational-models-and-simulation-associated#:~:text=Computational%20Modeling%20and%20Simulation%20,. [Accessed 15 Apr 2025].
[7] E. Campos-Nanez, “Metabolic modeling, simulated populations, and in-silico preclinical trials in type 1 diabetes,” 14-15 Sep 2016. [Online]. Available: https://nc3rs.org.uk/sites/default/files/documents/Workshop_reports/maths/10%20-%20Metabolic%20modeling,%20simulated%20populations,%20and%20in-silico%20preclinical%20trials%20in%20type%201%20diabetes.pdf#:~:text=type%201%20diabetes%20Dr%20Enrique,approval. [Accessed 15 Apr 2025].
[8] E. Neufeld, B. Lloyd, B. Schneider, W. Kainz and N. Kuster, “Functionalized Anatomical Models for Computational Life Sciences,” Sec. Computational Physiology and Medicine, vol. 9, 15 Nov 2018.
[9] N. Abboud, “FDA Unveils Transformative Guidance for Computational Modeling in Medical Device Submissions,” Thornton Tomasetti, 07 Feb 2024. [Online]. Available: https://www.thorntontomasetti.com/news/fda-guidance-computational-modeling-medical-device-submissions#:~:text=This%20CDRH%20guidance%20document%20is,use%20both%20evidence%20streams%20in. [Accessed 16 Apr 2025].
[10] FDA, “Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions,” [Online]. Available: https://www.fda.gov/media/154985/download#:~:text=%281%29%20Question%20of%20Interest%20,1%29%20Code%20verification%20results. [Accessed 15 April 2025].
[11] FDA, “PMA for Endurant II Stent Graft System,” 29 September 2017. [Online]. Available: https://www.accessdata.fda.gov/cdrh_docs/pdf10/P100021S063B.pdf. [Accessed 01 May 2025].
[12] Medtronic, “Medtronic’s Endurant II/IIs Stent Graft System Receives CE Mark For Use With ChEVAR Parallel Graft Technique,” 9 December 2016. [Online]. Available: https://www.medicaldesignandoutsourcing.com/medtronics-endurant-ii-iis-stent-graft-system-receives-ce-mark-for-use-with-chevar-parallel-graft-technique/. [Accessed 16 May 2025].
[13] FDA, “De Novo Classification Request for FFRct V. 1.4,” 6 November 2013. [Online]. Available: https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN130045.pdf. [Accessed 1 May 2025].
[14] HeartFlow, “HeartFlow FFRCT Analysis,” [Online]. Available: https://radiology.healthairegister.com/products/heartflow-ffrct-analysis/. [Accessed 16 May 2025].
[15] Smith and Nephew, “EVOS◊ SMALL Plating System,” [Online]. Available: https://www.smith-nephew.com/en/health-care-professionals/products/orthopaedics/evos-small. [Accessed 22 Sep 2025].
[16] Zimmer Biomet, “Oxford® Cementless,” [Online]. Available: https://www.zimmerbiomet.com/en/products-and-solutions/specialties/knee/oxford-cementless.html. [Accessed 16 May 2025].
[17] DePuy Synthes, “ATTUNE® Cementless KneeSystem,” [Online]. Available: https://www.jnjmedtech.com/en-NZ/product/attune-cementless-knee-system. [Accessed 23 September 2025].
[18] Arthrex, “Eclipse™ Stemless Shoulder Arthroplasty System,” 29 January 2021. [Online]. Available: https://www.arthrex.com/resources/VID1-000700-en-US/eclipse-stemless-shoulder-arthroplasty-system. [Accessed 16 May 2025].
Nathan Muller is a StarFish Medical Mechanical Engineer – Analysis and Design. His focus is in simulation engineering using computational modelling. As part of a design and development team, he frequently leads the development of mechanical design and device integration across disciplines, including targeted optimization and derisking activities through computational modelling and simulation (CM&S).
Images: StarFish Medical
Related Resources

Nick and Nigel walk through how sterile disposables are processed and verified before they reach the field.

The FDA agentic AI is making headlines after the agency announced its own internal AI review tool. In this episode of MedDevice by Design, Ariana and Mark discuss what this could mean for medical device submissions and regulatory efficiency.

The sandwich ELISA assay is one of the most common ELISA formats used in diagnostics. Nick and Nigel walk through the method step by step using simple visuals and plain language.

For manufacturers of novel devices that can make a significant impact to patient health, the goal of the program is to offer a path to streamlined and potentially faster market entry without sacrificing the rigour around ensuring safety and performance.
