Developing a Diagnostic Product for Use Outside a Laboratory
The COVID-19 pandemic has increased diagnostic testing outside the confinement of the laboratory. The hope is to enable healthcare professionals to perform testing in places where it was previously unthinkable to sample patients and directly analyze test results. Such places now include your home, airports, or the workplace. These innovative solutions greatly reduce the time to get a test result and are revolutionizing diagnostic screening. This blog outlines some of the considerations that go into developing a diagnostic product intended for use outside a traditional laboratory.
It excludes the category of products focused on at-home sampling with samples being shipped back to a centralized laboratory for analysis. (i.e. a saliva sample or nasal swab that is put in a package and shipped to the laboratory). Instead, the blog covers diagnostic products for which the entire workflow, including the analysis, is performed outside a centralized laboratory.
Waived vs Point-Of-Care Testing (POCT)
The term POCT can be confusing, but is used by FDA to describe an analytical method that is performed remotely away from a centralized laboratory facility. POCT can be complex and sometimes requires users to be professionally trained healthcare professionals. An additional rating by the Clinical Laboratory Improvement Amendments (CLIA) determines if a product is designated highly complex, moderately complex or waived. Waived testing is referenced by CLIA as simple tests that carry a low risk for an incorrect result. Products that are performed at home, airports or directly in the workplace, are generally waived POCT.
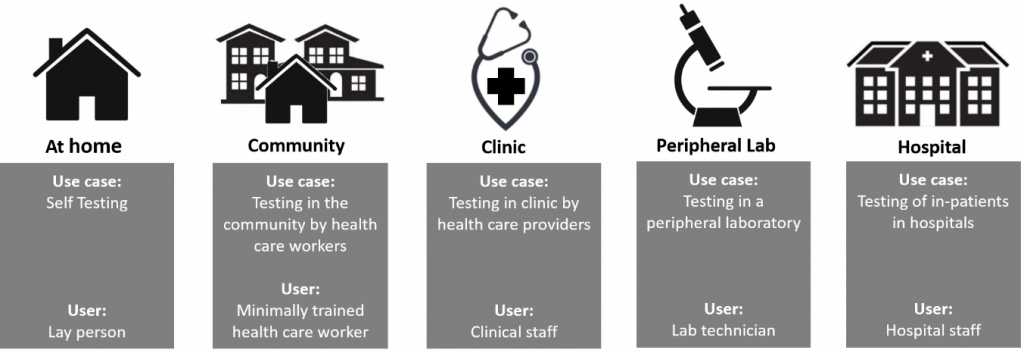
The term Point of Care applies to a continuum of use cases spanning near-patient testing in the hospital (e.g. Cath lab), to running a test by a health care professional in a clinic or pharmacy to performing a diagnostic procedure at-home (see figure below).
Determining where a POCT application fits in this continuum helps define design requirements and the accompanying need for summative usability testing.
The Intended User
The diagnostic device design process always starts by generating a target product profile (TPP). One of the key pieces of information in this TPP is an accurate definition of the intended user(s). In order to be designated as a waived product, the design authority will have to show that the likelihood of a false result is negligible after use or misuse by the intended user. This means that the product and its entire workflow (including sampling) are extremely robust and can be performed with minimal training/instructions.
Waived products often require minimal handling and, if the user accidentally mishandles the product or workflow, system controls should prevent the user from getting a false result. A typical example of a system control would be a reference measurement that is run in parallel to the actual test indicating that the test has been performed correctly (i.e. the control line on a lateral flow pregnancy test). As much as possible, these system controls should prevent a user from getting a false result. The regulatory submission should include evidence of extensive usability studies with a range of intended users. Part of the formative and summative usability assessments are “flex studies” in which the system is misused on purpose engaging system controls to mitigate specific user errors. Summative usability study reports must be submitted in addition to the standard clinical program submissions to demonstrate safety and efficacy.
The Use Environment and Product Labelling
Typically, humidity, air pressure, lighting, available freezer storage and temperature of the environment in which waived tests are performed are not controlled to the same degree as a centralized laboratory facility. Constraints that affect performance for a variety of environmental conditions need to be explored for proper product labelling. Also, verification and validation studies need to cover claims for environmental conditions or edge case scenarios.
Regulatory bodies will authorize the use of POCT devices in a particular environment which is reflected in the device labeling. For device manufacturers, one approach that is often used successfully is to first target use environments with lower regulatory burdens (e.g. targeting use in a clinic before targeting at-home use). The remaining data that is required for the “at-home” designation can then be gathered while the device is already on-market. The device labelling can be adjusted after regulatory approval for the new use case is obtained.
Enabling technologies and product design
Diagnostic assays in the lab often involve pipetting and the use of dedicated laboratory equipment and consumables (e.g. centrifuge, microcentrifuge tubes or 96-well plates, incubators, etc.). For POCT applications, pipetting as a means of transferring liquids, is ideally “designed-out of the system” and standard laboratory equipment is not available outside the laboratory. Form factors that are widely used to house an entire diagnostic procedure in a compact form factor are lateral flow technologies and microfluidics. Selection of the most appropriate technology depends on what needs to be detected in the assay (antibody, antigen, nucleic acids, etc.) or the application (serological testing, antigen detection, molecular testing, etc.). Generally, the use of lateral flow technologies is limited to antibody or antigen detection whereas microfluidic approaches can be used for detection of all biological markers.
Contamination, Biohazard and Disposal
The non-laboratory environment often does not have a sterile workbench for performing diagnostic tests. Packaging of consumables helps keep sampling devices sterile until use (e.g. swab or spit cup, etc.) to prevent environmental contamination from entering the system and invalidating results or; even worse, altering results. A closed system (meaning that the sample is placed into the system and no further handling of the sample is possible), greatly reduces the risk of contamination. Having a closed system also helps prevent cross-contamination of the environment by the diagnostic test itself. Molecular tests generate a lot of biological material as part of the amplification process that takes place during the testing procedure (e.g. PCR, isothermal amplification). A closed system makes it less likely for these testing products to escape and contaminate a testing location.
Another consideration is what to do with the test after use. Generally, non-laboratory environments do not have a biohazard waste stream. Preferably, solutions for biohazard disposal are part of the product design; for example, by including an integrated decontamination step that is engaged after the test has been performed.
Shelf life and packaging
Another aspect in the design process that needs to be considered early in the development process is the shelf life of each system component. For shorter shelf life claims, accelerated stability programs can be used, however; a shelf life claim that is longer than six months will need to be substantiated with a real-time shelf life program. It is important to consider this early in the development cycle and allow time for stability programs to take place.
Lyophilisation (freeze-drying) of biological reagents helps make reagents stable at room temperature for shipment and long term storage which is critical for most POCT. Lyophilisation adds some technical complications to the manufacturing process and lyophilized reagents need to be packaged and stored under low humidity conditions. Also, reagent formulations frequently have to be modified to make them compatible with lyophilisation. That is why lyophilisation is generally designed into biological reagents as part of Design for Manufacturing (DFM).
Adequate protection of sensitive system components during shipment, storage and use can greatly enhance performance in the field. The packaging can help protect components from light, humidity, temperature fluctuations, and shock. It can also provide a sterile barrier and guide proper use in the field. Ideally, product validations and the shelf life programs are all performed using the final packaging configuration, so designing packaging early in the development cycle helps extend shelf life, aids clinical validation and formative and summative usability testing.
Data handling, interpretation and data storage
After the test has been run, the data needs to be interpreted. The complexity of data interpretation is immediately linked to the product’s ease of use and is a major focal point for regulatory bodies. Some tests provide a “black-and-white” qualitative assessment, but for many diagnostics, data interpretation is done by a software algorithm after which the results are communicated to the user. The underlying algorithm that facilitates interpretation can be complex and frequently has ties to Quality Control (QC) measures that are part of manufacturing. It is difficult to change algorithms after a product has been released so it is critical that algorithms undergo extensive testing and fine-tuning covering lot-to-lot variability, different patient profiles, users and consumables to determine appropriateness of the algorithm for all potential use cases and circumstances.
Finally, the data that has been generated can be pushed to the cloud to allow easy tracking of health related metrics. This may be commercially attractive for product developers, however; data handling needs to be compliant with Health Insurance Portability and Accountability Act (HIPAA) regulations. How POCT results are best communicated to the patient is an ethical question as much as a technical question. What are the implications of providing health related results without necessarily having a health care professional delivering the message or providing follow up consultation? The appropriate course of action depends on the application but thoughts on how to deal with data handling need to be formulated early as they can tie in directly to the regulatory approach.
Conclusion
In general, taking a diagnostic test and designing it for use outside a laboratory requires an extensive re-design of the lab-based product. In addition to characteristics like price, time-to-result and portability, thoughts on ease-of-use, stability, robustness, disposal of consumables and data handling should be considered early on. Well executed, waived POCT provide tremendous value in elevating the quality of care and patient experience in non-clinical settings. Undoubtedly, in upcoming years, a lot more lab-based diagnostic applications will be re-designed for POCT use.
Photo 208516281 / Test Covid © Amanda Lewis | Dreamstime.com
Joris van der Heijden is Bio Services Program Manager at StarFish Medical. Previously he was Director of R&D at Spartan Bioscience where he led the development of a point-of-care COVID-19 diagnostic test. Joris received his PhD in infectious diseases from UBC.