
How Computational Modeling and Simulation Accelerates Medical Device Innovation
The costs of early-stage medical device development in North America and Europe continue to rise. Increasing technical complexity and the compounding costs of nonclinical and clinical evaluations are driving this trend. In this environment, Computational Modeling and Simulation (CM&S) is becoming an essential tool to reduce development risk and cost.
Through techniques such as finite element analysis (FEA) and computational fluid dynamics (CFD), CM&S enables engineers to analyze device behavior virtually—accelerating design iteration, lowering risk, and reducing overall development cost.
This article defines CM&S, explores common use cases, and highlights high-value areas where CM&S strengthens modern, cost-effective product development strategies.
What Is CM&S in Medical Devices?
CM&S uses mathematical simulations to replicate the physical behavior of systems. In medical device development, these models predict how products will perform under various operating conditions—whether structural stress on an implant, heat dissipation in a surgical tool, or blood flow through a catheter.
Key modeling methods include:
- Structural FEA
- Thermal Analysis
- CFD
- Multiphysics Modeling
These tools generate virtual simulations to evaluate device performance and guide design decisions.
Common CM&S Use Cases in Medical Devices
CM&S is used across a wide range of medical devices and clinical applications. Examples include:
- Implantable Devices: Assessing structural integrity under cyclic loading
- Surgical Tools: Modeling ergonomics, thermal performance, and mechanical durability
- Drug Delivery Systems: Simulating dosing rates, pressure requirements, and reservoir dynamics
- Diagnostics Platforms: Predicting temperature profiles or fluid interactions
- Microfluidics: Optimizing flow channels, mixing behavior, and reagent delivery
Six High-Value Areas Where CM&S Accelerates Development
Faster Development Cycles
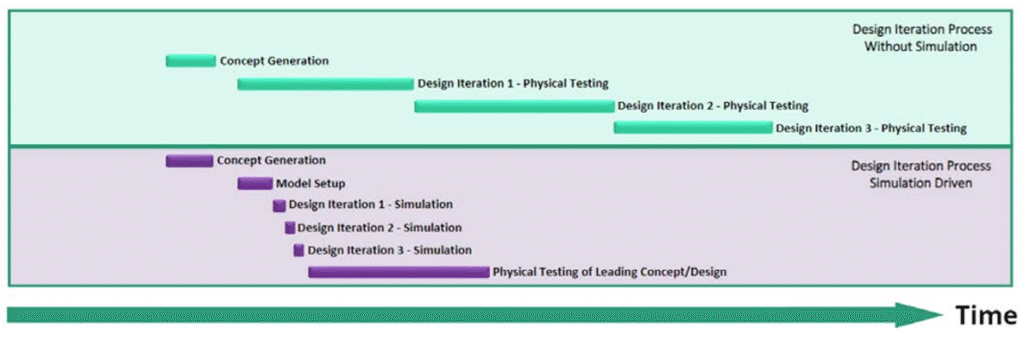
Traditional development relies on iterative physical prototyping—a slow and costly process. CM&S enables teams to test dozens of virtual concepts before committing to physical prototypes.
For example, modeling microfluidic cartridge flow dynamics using CFD software can identify bottlenecks or dead zones—without dispensing a single drop of fluid. What once took weeks or months can now be achieved in hours or days.
Smarter, Data-Driven Design Decisions
Simulations generate detailed data to guide design decisions. Predictive insights into stress, temperature, and motion allow engineers to optimize designs and prevent failure modes.
In structural FEA, for instance, stress concentrations in a surgical clamp can be identified and addressed—improving durability without costly prototyping.
Reduced Cost and Waste
Physical prototyping is expensive—not just in materials, but in labor and time. CM&S minimizes prototype iterations by resolving issues virtually. This reduces failed tests and late-stage redesigns, saving significant costs across the development cycle.
Early Risk Mitigation
CM&S allows teams to simulate worst-case scenarios—some of which are difficult or impossible to recreate in bench tests.
These simulations support faster, safer risk assessment. Early insights enable inexpensive adjustments and reduce downstream risk—both during development and post-market.
Regulatory Submission Confidence
Regulatory bodies such as the FDA are increasingly accepting simulation data as part of risk assessment. The FDA’s Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions guidance highlights the value of CM&S in regulatory filings.
A key element is the Risk-Informed Credibility Assessment Framework (RICAF), which guides model verification, validation, and uncertainty quantification (VVUQ). Well-documented simulations can supplement or replace some bench tests—saving time during verification and validation.
Alignment with Industry Standards
Confidence in model predictions is critical. The ASME V&V 40 standard provides a structured approach to model validation:
- Conceptualization
- Data generation
- Uncertainty quantification
- Comparison with bench tests
This framework ensures models reflect real-world performance. It also promotes sensitivity analysis to identify key parameters and justify safety margins. Early detection of weaknesses improves both computational and bench testing, reducing the risk of costly redesigns.
Conclusion: Smarter Design Starts with Smarter Tools
CM&S is a powerful enabler of smart, lean, and efficient medical device development. It helps teams move faster, make informed decisions, reduce costs, and de-risk innovation.
By using advanced analysis tools such as Ansys, CM&S captures the complexities of fluidics, structural mechanics, and thermal systems.
At StarFish Medical, we bring deep expertise in simulation to support medtech innovators. Whether you’re developing next-generation diagnostics or refining surgical tools, we can help you leverage modeling to accelerate time to market.
TL;DR:
- CM&S accelerates innovation in surgical tools, implants, diagnostics, and more.
- CM&S reduces medical device development time and cost.
- Virtual simulations optimize design and identify risks early.
- Regulatory agencies increasingly accept CM&S data in submissions.
- ASME V&V 40 and FDA RICAF guide simulation credibility.
Jurgen Frasheri is an Intermediate Mechanical Engineer at StarFish Medical. While a student at University of Toronto, Jurgen belonged to an engineering design club that develops autonomous underwater vehicles for international design competitions.
Images: StarFish Medical
References
[1] Centre for Devices and Radiological Health, “Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions,” November 2023. [Online]. Available: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-credibility-computational-modeling-and-simulation-medical-device-submissions. [Accessed 29 May 2025].
Related Resources

Nick and Nigel walk through how sterile disposables are processed and verified before they reach the field.

The FDA agentic AI is making headlines after the agency announced its own internal AI review tool. In this episode of MedDevice by Design, Ariana and Mark discuss what this could mean for medical device submissions and regulatory efficiency.

The sandwich ELISA assay is one of the most common ELISA formats used in diagnostics. Nick and Nigel walk through the method step by step using simple visuals and plain language.

For manufacturers of novel devices that can make a significant impact to patient health, the goal of the program is to offer a path to streamlined and potentially faster market entry without sacrificing the rigour around ensuring safety and performance.