Recently we conducted pre-testing (testing done in-house to increase confidence before undertaking more expensive formal testing) for ISO 18562-2:2017 Biocompatibility evaluation of breathing gas pathways in healthcare applications — Part 2: Tests for emissions of particulate matter is an ISO standard for particulate testing in breathing gas pathways. This is a sub-standard of the larger ISO 18562-1:2017 Biocompatibility evaluation of breathing gas pathways in healthcare applications — Part 1: Evaluation and testing within a risk management process standard. In this blog I will share our approach from my engineering perspective.
Why 18562-2:2017 particulate testing is important
ISO 18562-2:2017 is mandatory as part of the overall ISO 10993-1:2018 Biological evaluation of medical devices — Part 1: Evaluation and testing within a risk management process biocompatibility assessment[1]* for gas path medical devices. It’s important because particles have been shown to be hazardous to patients’ respiratory and cardiovascular health[2]. Minimizing particles is essential to ensure the benefits of a gas path medical device outweigh the risks.
Not all medical devices require evaluation against 18562:2-2017
Only breathing gas pathways of medical devices and accessories require 18562-2:2017 testing[3]. A breathing gas pathway is defined as an interior surface over which fluids flow that can then be inspired[4]. In this case, that applied to us.
Evaluating if testing is required. Not all gas path devices require physical testing. A paper-based evaluation can be done to determine whether or not to proceed with testing by considering the factors listed below and comparing those to the risk management file[5]. In our case, the device we were developing had a number of risks that led us to determine we needed testing.
- Process of reprocessing (does reprocessing or lack thereof add risk?). How is the device cleaned, and how often? Does the cleaning process introduce particles?
- Worst case patient exposure (more exposure is usually more risky). For example, the device may be only intended for a single, partial breath, or for long term use and exposure to particles will be different with different risk associated with use.
- How simple is the device? Are there moving parts? (complex devices with moving parts are usually considered higher risk and therefore often require more thorough testing[6]).
- Is there a standard patient particle filter in close proximity of the patient? (if so, the likelihood of particulate exposure to the patient may be minimal if the filter ‘cleans’ the air).
- Are the materials, processes, and/or cleaning procedures similar to other approved devices and therefore providing confidence that the device in question is safe?[7] (if they are similar or exactly the same, it may be easier to justify non-testing with existing data).
Plan for Pre-testing. Typically, a 3rd party testing laboratory will conduct formal testing. However, pre-testing ahead of time to increase confidence that it will pass the 3rd party test on the first submission, or as part of the R&D process. The following describes how we did pre-testing.
Choose device version carefully. It is important to use the final version of the device, i.e. the version that is being manufactured, for final testing. If that’s not practical, be prepared to describe and justify the differences between the tested version of the device and the final finished device, including not only the design/materials, but also the cleaning/handling and assembly process.
Decide how to measure particulate. Typically, this is done with a set of filters on the outlet to collect particles. The filters are weighed before and after outlet gas is run through them. Particulate should be collected at a number of time points during device use – e.g. starting at 0hrs, after 1 or 2 days, and after a number of days, depending on how long the device is intended to be used and if the device is expected to have a changing level of particulate matter over time[8]. The more data points collected, the lower total particulates there should be because more data points allow for a more accurate assessment, and assuming the typical scenario, more accuracy should result in a lower particulate count.
Determine how to set everything up for pre-testing. Here are some key factors that we considered as they were important for our specific device’s pre-testing:
- How to connect to the filter, and how those connecting parts might affect the results by contributing to particle count.
- Assessment of the device’s built-in inlet filter mesh size (if there is one) to see if it will remove particles in the ambient air. If the mesh size is too large (or there isn’t a built-in inlet filter) a double filter configuration (one on the inlet and the outlet) may be needed.
- Assessment of the device’s built-in outlet filter mesh size (if there is one) to see how that might affect results and if the sample should be taken before or after.
- Consideration of back pressure and its effect on the device’s throughput. For example, testing a ventilator meant to deliver 20L/min through a 0.2um filter with a small cross-sectional area may significantly increase back pressure or reduce flow rate which may affect the way a device functions or invalidate the results. This needs to be mitigated (often by adding a number of filters in parallel) or otherwise accounted for (often by measuring output air flow directly).
- Also consider if a backpressure device is needed to ensure the system functions correctly. For example, some ventilators will alarm or work differently if they don’t have enough back pressure.
- Ensure there is an adequate mass scale with a very fine precision, and/or drying equipment (see the section on Measurement below).
- Ensure the filters won’t get so wet that they become less effective[9].
- Consider if the device utilizes multiple types of gas (e.g., can switch between air and oxygen), and if so then both paths should typically be included in testing.
We asked ourselves: what filter sizes should we use? The graphic below shows the ranges of particles that must be addressed. This leaves options: 1) use 0.2um filter(s) only, which is the simplest but is very conservative; 2) use a 2.5um filter followed by 0.2um filter(s), which will give an accurate result for the smaller particle range but will give a conservative result for the larger particle range; or 3) use a 10um filter, followed by a 2.5um filter, followed by 0.2um filter(s) for the most accurate results.
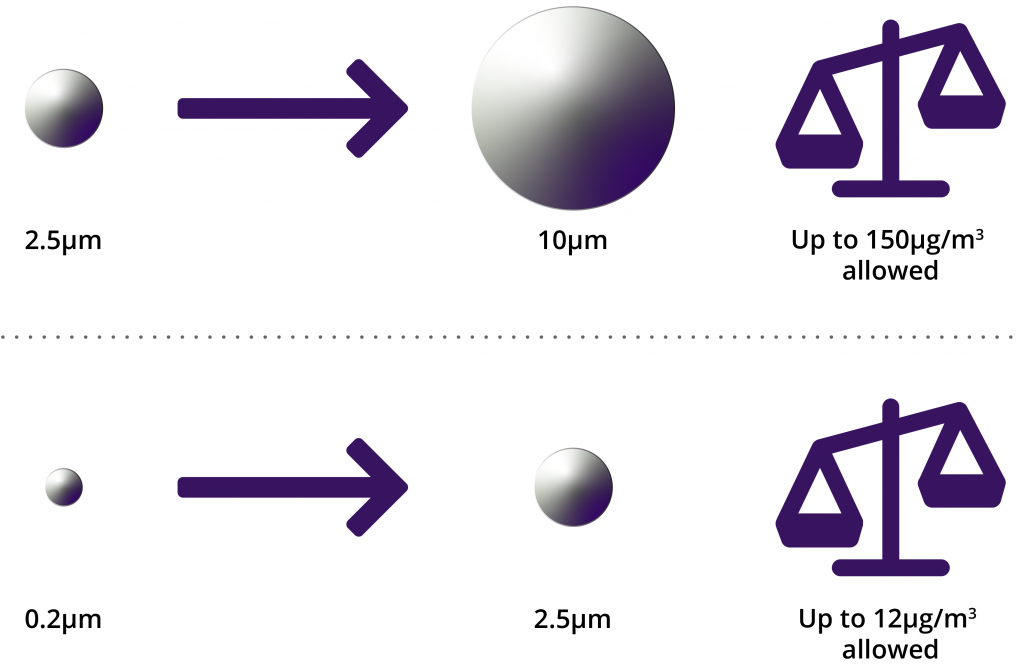
10um > Particle size > 2.5um, allowable limit is 150ug/m3
Consider if any other factors might impact results. It’s advisable to consider all factors that could impact results, and set those to the worst case. This may involve adjusting settings for speed, intake, or accessories. Inlet air, and room temperature may also affect results, as well as foreseeable user error.
Lay out a test plan to inform the testing details. This resulted in the following decisions:
- Use a device that has completed final assembly (if that includes packaging, then the device should be packaged as well).
- Dry the filters, then measure their mass before testing (when using a 10um filter, this doesn’t need to be weighed as particles over 10um aren’t considered in 18562—2:2017).
- Consider adding an additional spare filter(s) as a control to ensure all other factors, such as scale accuracy and humidity are accounted for. For example, if the control filter has gained or lost significant weight for no apparent reason, there might be an issue with the setup.
- For simple systems, simply hook the filter set up to the outlet. If necessary, also hook a filter up to the inlet (see the section on Setting Things Up above).
- Run the system for a length of time to pass a sufficient amount of air through such that you will get a meaningful result based on the accuracy of your scale. For example, if the scale can accurately measure in increments of 5μg, pass 2m3 through the filter (maximum passing weight = 24ug, or at approximately 5x the accuracy of the scale) to get a more stable result for the smaller particle range[10].
- For some devices, the maximum amount of gas a patient may ever be exposed to may be less than the required volume calculated above. In this case, connect a number of devices to the same filter to increase the potential particulate mass to detectable levels.
- Any inlet (as inlet air quality is not in the scope of 18562-2:2017) and 10um filters (as particles over 10um aren’t considered in 18562—2:2017) can be discarded.
- Dry, then measure the mass of the 0.2um and 2.5um filters after testing. Also do this to the control, if there is one, to confirm process effectiveness.
Are filters the only way to test? No. In fact, only one of four different approaches discussed in 18562-2 are noted here (the others being indirect sampling, inertial particle separation, and laser particle counting). The other approaches may be more suitable a specific device and should be considered carefully.
This article explained how we approached in-house pre-testing to add confidence to our upcoming 18562:2-2017 formal testing. I hope it proves useful to anyone who needs to do the same. Remember to refer to the standard as much as possible, and Happy Testing!
Gemelli trio-smart breath test
Commercializing revolutionary breath test technology
* Footnotes refer to page numbers in the downloadable ISO standard PDF available at https://www.iso.org/standard/62893.html
[1] Page 5
[2] Page 5
[3] Scope, and title of the standard
[4] ISO 18562-1 definitions, simplified though
[5] Page 9
[6] Section 5.2 paragraph 1
[7] 18562-1 page 6
[8] Sec 5.8
[9] Page 7
[10] Page 6
Nigel Syrotuck is a StarFish Medical Team Lead Mechanical Engineer and frequent guest blogger for medical device media including MD+DI, Medical Product Outsourcing, and Medtech Intelligence. He works on projects big and small and blogs on everything in-between.