We’ve become so used to stainless steels being stain-free, that we are shocked when we see rust on them. While the conditions required for stainless steel rusting are more likely to occur in medical lab equipment where stainless and salt solutions come together, it can happen in any device, including implants:
A first thought is often that we’ve set up a galvanic cell, and our stainless is rusting as a direct result. But that may not be the issue. It’s important to look at the other corrosion mechanisms as they could be the result.
Crevice corrosion is one of those other mechanisms, but the resolution will be different. Crevice corrosion occurs when the surface of the stainless is oxygen deprived, as in a joint. A slight gap, even those due to manufacturing tolerances, can create a region where fluid can accumulate, but is stagnant. Oxygen in the fluid is reduced over time and chlorides are allowed to build. These chlorides form acids which attack the stainless. The stainless does not need a second metal – it just needs a small gap and the right solution. Pitting can be severe in these cases, and can be difficult to solve. Geometry can be altered to change remove the crevices or the manner in which fluid can pool, but sometimes the resolution may be to change to another metal like titanium which resists the chlorides (beware of higher temperatures), or to a plastic.
In contract, galvanic corrosion is caused by an electrochemical cell created where reduction and oxidation (redox) reactions are occurring. The cell needs three equally crucial constituents: an electrolyte, two dissimilar metals, and all three in contact each other. The resulting cell creates electrical potential which can be strong enough to cause oxidation of one of the metals (the anode).
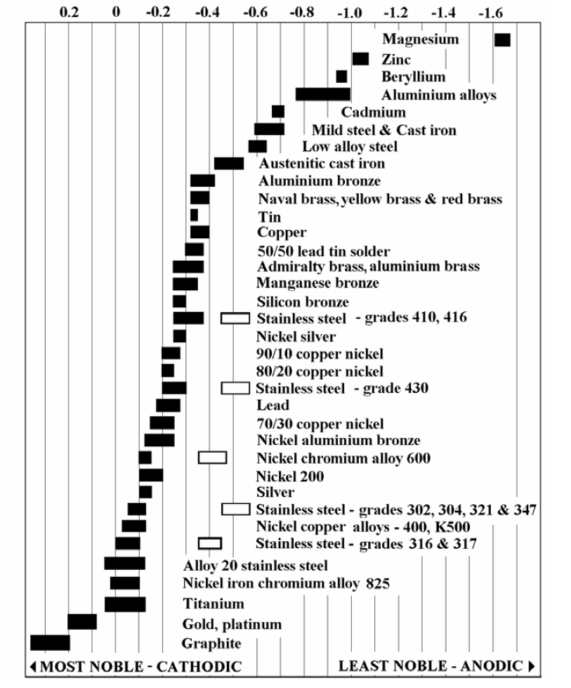
Stainless steels are stainless because they create a small, passive barrier which has a high affinity for its electrons. It is usually more cathodic than metals most commonly attached to stainless. See Figure 1: Galvanic Table of various metals above.
In other words, it’s usually the effect of stainless on the other material that’s the problem. Aluminium, for example, will have some serious pitting issues in a saline solution. But it’s possible that the stainless may be corroding, in which case matching metals will be required to solve the issue. The rule of thumb is that if you can limit the potential difference to 0.25V, galvanic corrosion will be negligible. However, you may need to limit the potential difference to 0.1V for particularly harsh environments. (Note – removing the electrolyte will also stop the corrosion!) You will often see two regions for a given stainless steel: an “active” and “passive” region. Active stainless is where that passive barrier is abraded or otherwise not allowed to form.
The last point I’ll mention is contamination from manufacturing. Small particulates, plain steel for example, become embedded in the surface and cause surface staining on the stainless. If the part is machined on a CNC machine that also does steel parts, small particulates of steel can contaminate the coolants, and when the stainless part is machined, become embedded in the surface. Similarly, buffing wheels that have been used on steel parts and then on stainless can similarly embed steel particulates, as can other steel tools like wrenches. It’s these non-stainless particles that are rusting and causing the surface staining. Check with your machining house, and ensure that they are not cross-contaminating your stainless parts. For sensitive applications, electro-polishing can be used to resolve the issue, which can also improve the finish and abrasion of the stainless as well.
Small iron nodules could also be within the stainless steel matrix itself if the steel was processed incorrectly. Even if the grain structure is not a structural issue but purely cosmetic, it’s nonetheless unacceptable. Metallurgical analysis may be required to determine the exact source, whether within the grain structure, or surface contamination.
Stainless steel is a fantastic material, but stainless doesn’t mean stain-proof. There are a number of other causes for corrosion such as inter-granular corrosion (usually due to poor welding techniques), or microbial staining, and there is lots of literature out there for all of these corrosion mechanisms. The first step is understanding the many causes of corrosion.
Dana Trousil is a StarFish Medical Mechanical Engineer. He has successfully launched many products, from small volume production up to moderate volumes (up to 1 million parts per year). He is a firm believer in moss not growing on a rolling stone.
Images: StarFish Medical