Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
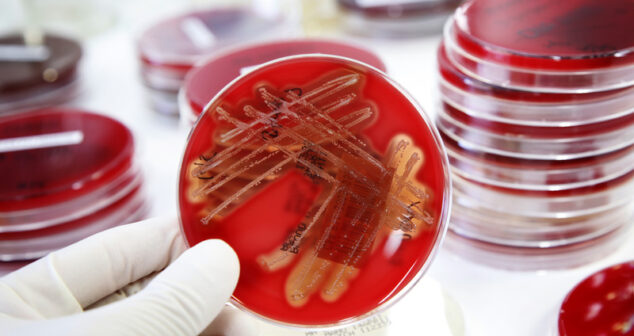
Design engineer’s perspective of medical device biocompatibility to help determine if a device needs to be biocompatible.
-

Highlights of lessons learned from 3 FDA inspection strategies and visits to medical device manufacturer and clients.
-

FDA inspection outcomes from 3 very different inspection preparation strategies with lessons learned from the inspections.
-

Adaptive studies can be especially useful in the pivotal stage if there are uncertainties about one or two aspects of the study.
-

FDA final rule on symbols is an important step towards global medical device harmonization and hopefully for a decrease in manufacturing costs as well.
-

Review of sections 4, 5 and 6 of ISO 13485:2016 and the major changes from the 2003 version with potential impacts on QMS.
-

FDA inspection of our Quality Management System (QMS) and 2 medical devices that are contracted to StarFish Medical manufacturing.
-
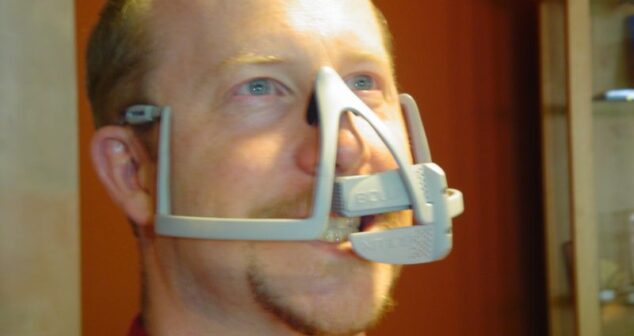
Exploration of applying IEC-62366-1:2015 or human factors engineering to the design of medical devices compared to the FDA guidance document.
-
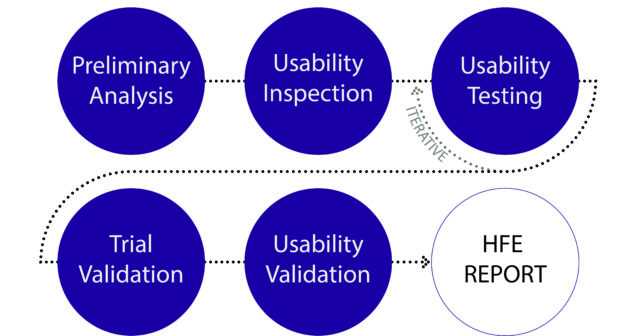
Did you know that this year FDA's Human Factors Premarket Evaluation Team have a priority review hit-list for medical devices?