Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Four areas that can make or break a new medical device development project from the start. Experts identify actions and offer advice that helps ensure new medical device projects start on the right foot.
-

Summary of nine-step process for developing and assessing the credibility of CM&S for regulatory submissions in the FDA Guidance Document “Assessing the Credibility of Computational Modeling and Simulation (CM&S) in Medical Device Submissions (17th November 2023)”.
-

Opening a biomed lab includes managing multi-layer organizational involvement all the way from facilities to the head of operations. When starting a new lab from scratch, the first step is to fully understand the organization’s mission and business goals. This will impact how you approach setup and any purchasing decisions you make. This article describes 12 essential steps to set up a biomed lab.
-

In this episode of Bio Break, Joris van der Heijden and Nick Allan discuss the critical role of specialized facilities in the successful development of medical devices. From testing laboratories to clean rooms, they explore the infrastructure needed to support innovation and ensure safe, effective products reach the market.
-
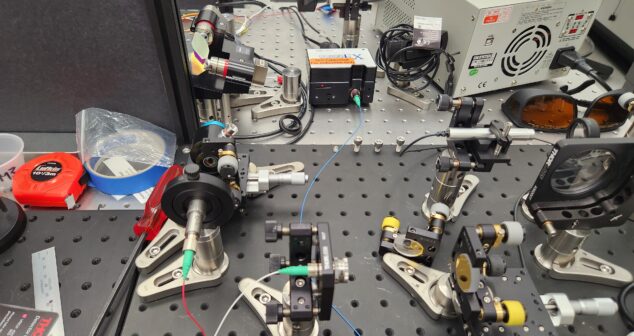
Tips for building a medical device optical breadboard prototype. When designing a medical device made of complex, multi-functional mutually interacting subsystems it’s best to de-risk those subsystems instead of coming up with a paper design, assembling it, and hoping it works first time. De-risking individual subsystem behaviours one at a time enables more-focused assessments and improves troubleshooting.
-

Speed to market is a crucial part of medical device success. Experts draw upon their experience with hundreds of medical device products for proven tips and recommendations that increase medical device speed to market without sacrificing safety or quality.
-

Engineers, regulatory, manufacturing and optics experts share their experiences and lessons learned commercializing hundreds of medical devices with optics components and interacting with optics engineers.
-
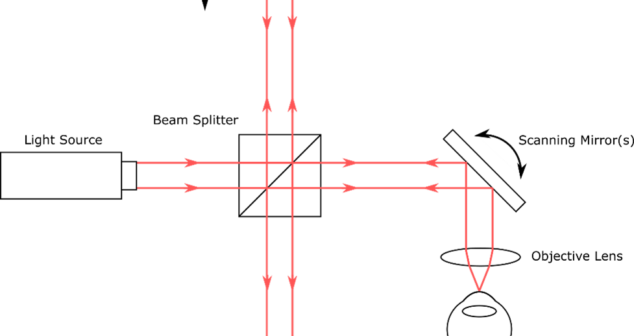
Overview of Optical Coherence Tomography (OCT) Types includes some advantages and disadvantages of the imaging techniques in medical devices.
-

Whether you're in the early stages of a new project or refining a product concept, this episode of Bio Break delves into the foundational importance of a well-defined Target Product Profile (TPP) in medical device development. It's packed with practical advice and expert insights to set you on the path to success.