Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

MDSAP enables Medical Device companies to save in Registrar’s fees, eliminate duplication of inspections and gain faster access to markets.
-

Reduce costs by reusing IVD Verification Test equipment for verification of optical components as part of manufacturing quality control.
-

Regulatory affairs expert explains how to write a best practice ISO 9000 Medical Device Quality Manual using 3 simple rules.
-
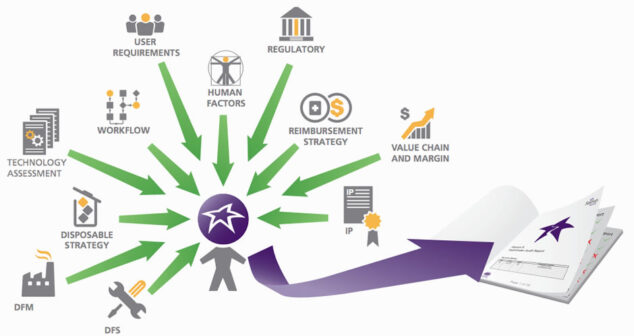
Medical Device Pathfinder process for Intellectual Property, Consumables Strategy, Value Chain and Margin, and Reimbursement.
-

The best way to avoid waste is to consider medical device regulatory requirements at a project outset.
-
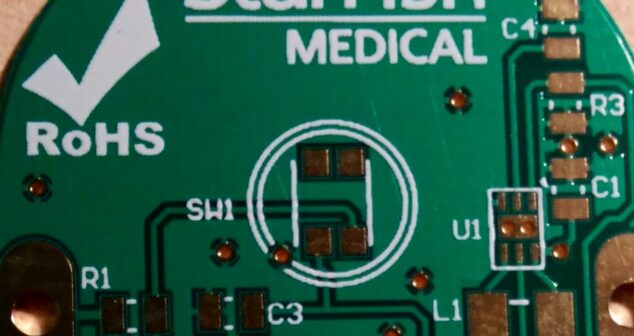
RoHS 2 and Medical Devices impact design and manufacture. Failure to meet requirements could result in product recalls.
-

Reader Favorites: 10 Most Read StarFish Medical Device Blogs of 2013. From FDA Guidelines to EMS pre-screening to Medical Device Margins.
-

How do we define the quality of a medical device? What attributes cause us to come to the conclusion that a device…