Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
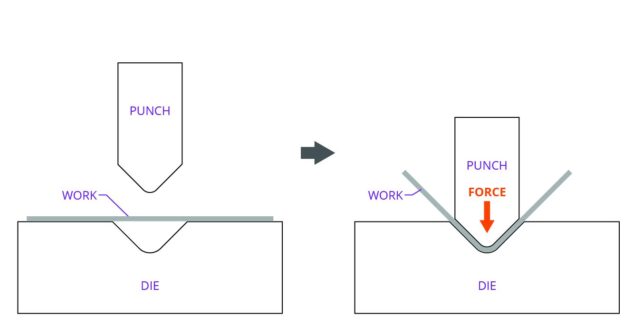
Sheet metal fabrication is an excellent method of making mechanical parts. This article explains how to avoid hole distortion in sheet metal parts.
-
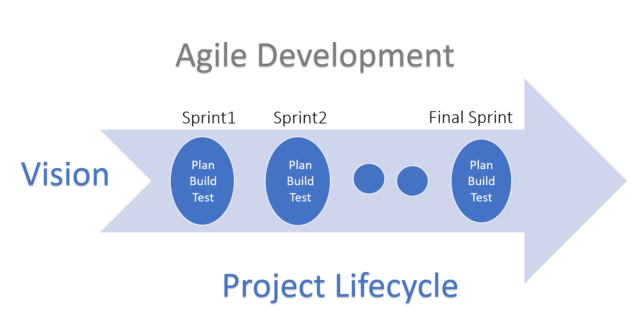
Agile methodology in Medical Devices Product Development methodology is supported by an FDA guidance and IEC standards.
-

Article offers 10 Ultra Low Power Embedded Design tips to meet growing demand for medical devices that do more with less power.
-
Five tips and tricks for ElectroMagnetic Compatibility (EMC) testing, a key part of medical and other device development.
-

9 medical device commercialization resolutions recommended by our team of engineers, designers, QA/RA, and manufacturing professionals.
-
Watch the most viewed StarFish Medical videos of 2018 to learn about medical device design, development, regulations, and manufacture.
-

New medtech blogs and new authors join our 2018 “most read” list. Regulatory was the most popular topic, followed by Electrical Engineering.
-

PFMEA prevents causes rather than detecting them (or the effects of failures), and should be incorporated into medical device design.
-
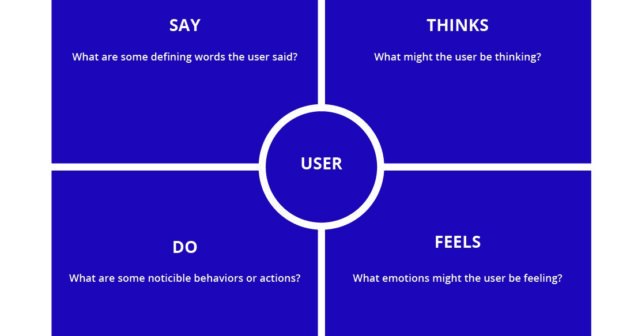
Empathic Design paying attention to what female users say, think, do, and feel, gives a holistic understanding of user desires and needs.