Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
Digital Health & medical device regulatory and technical implications: HIPAA, Mobile Medical App (MMA), Medical Device Data System (MDDS), and app stores.
-

Digital Health Advantages and Disadvantages describes numerous advantages and motivations for medical devices to engage with Digital Health.
-
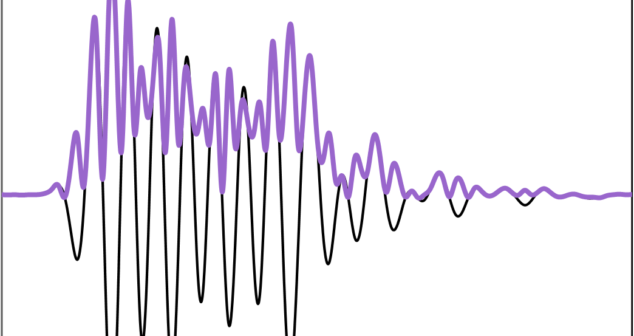
Ultrasound signal processing techniques cover a number of methods of demodulation. Pros and Cons of two that are predominant today.
-
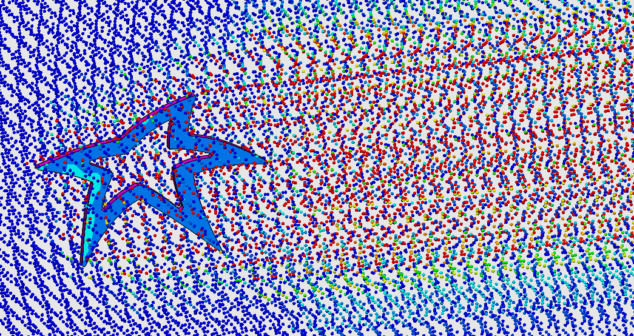
Medical device CFD simulations allow you to generate images and videos with little effort to predict and optimize fluid and thermal paths.
-

The tricorder is the ultimate objective of diagnostic medical device development. But is it Medical Device fact or Fiction?
-

StarFish Medical device development videos viewing is up 38% in 2014. Countdown of the Top 10 most viewed videos in 2014.
-

Most read StarFish Medical Device Development Blogs of 2014: regulatory information, analysis, testing, design, career path, and finances.
-

4 tips to collect meaningful user data for emerging medical device technology and take an appropriate level of risk and reward.
-

Medical Device Development tools to select the right concept and make more effective technical decisions using the Concept Downselect Matrix.