Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

On Demand recording of Automating the pre-commercial exit as a strategy for high-value startups presented by Amr Salahieh, serial entrepreneur and founder of Sadra Medical and Shifamed, at Medical Device Playbook Newport Beach 2024 is now available for on demand viewing.
-

Drawing on their extensive experience in executive roles and high-tech M&A, the speakers delivered an engaging discussion tailored for MedTech founders and executives.
-

In this episode of Bio Break, Joris van der Heijden and Nick Allan discuss an advanced category of combination devices: drug activation devices. Unlike drug delivery devices that transport medication to specific locations, drug activation devices ensure the drug becomes active precisely where it is needed in the body, reducing systemic side effects and enhancing therapeutic effectiveness.
-
Artificial intelligence (AI) has rapidly become a transformative force across industries, but its application in medical devices presents unique challenges. In his article "A Practical Future of Developing AI for Medical Devices" for MD+DI, Thor Tronrud, a Research and Data Analysis-Focused Software Engineer at StarFish Medical, sheds light on bridging knowledge gaps, navigating regulatory constraints, and developing robust AI solutions for medical devices.
-

The U.S. Food and Drug Administration (FDA) made a significant update to its regulatory approach for in vitro diagnostic products (IVDs), including laboratory developed tests (LDTs). Detailed in a FDA Laboratory Developed Tests Policy guidance document issued on June 25, 2024.
-
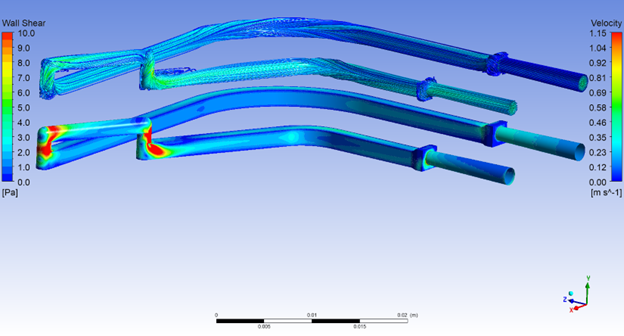
Drug Delivery Solutions for Cell and Gene Therapy (CGT) Challenges explores effective delivery devices and solutions for advanced CGT therapies and their unique challenges.
-
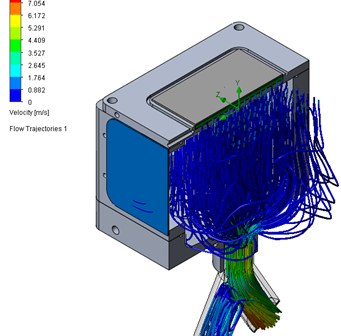
Aerosol Drug Delivery Systems, including Aerosol-based pulmonary-delivered drug devices, offer significant value by enabling targeted drug delivery directly to the respiratory system.
-
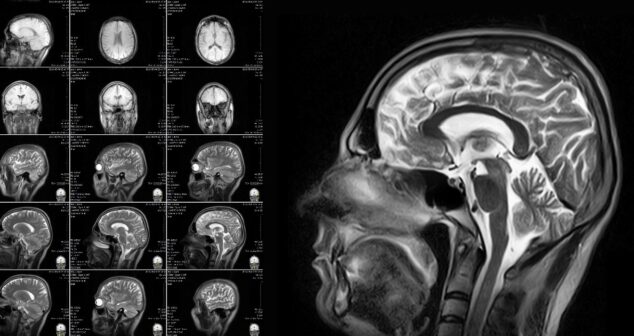
Drug Delivery System Imaging Technology options include MRI, CT, and Ultrasound. This article highlights advantages and disadvantages for each technology in guiding drug delivery.
-

Dive into the world of assay development with this informative episode of Bio Break, where Nick and Joris explore two critical concepts: Limit of Detection (LOD) and Limit of Quantification (LOQ). These terms might sound similar, but their implications for clinical diagnostics and medical devices are vastly different. Whether you’re an engineer, researcher, or product developer, this video sheds light on why both LOD and LOQ are vital in ensuring the precision and reliability of diagnostic tests.