Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Overview of battery safety for medical devices addressing design issues related to size, form, capacity and performance demands on batteries.
-

Ingress protection for home healthcare medical devices covers the 60601-1-11 collateral standard and what developers should know.
-

The amount of total company funding to develop a Class II 510(k) cleared medical device is approximately $30 million. The development and engineering costs comprise approximately $2-5 million of this total. This estimate is built upon a meta-analysis of various references as well as our experiences in engaging with companies. Many factors influence these costs, including the need for clinical studies, regulatory pathway, and technology complexity. Refinement of these numbers requires a professional team to understand the technology, regulatory, and business opportunities.
-
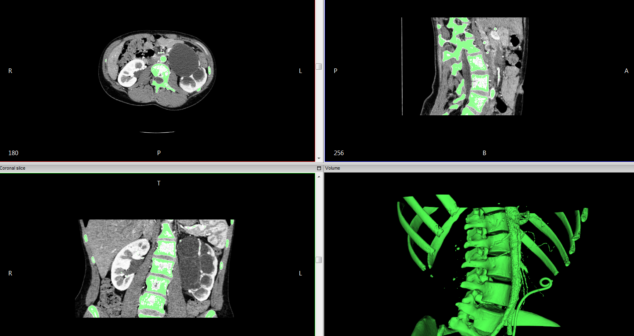
How to create accurate and functional 3D models from patient imaging data. Develop anatomically accurate prototypes for medical device design.
-

One of the key requirements for ISO 60601-1 is the protection against water ingress in Medical Devices. Three areas to watch are discussed in this article..
-

There are a lot of things to consider when creating a summative usability test. The first time can be overwhelming. What scenarios do I test? How do I identify a critical task? How many participants do I need? Do I need to test the training? In this eGuide I will help you avoid common mistakes, like using a formative test structure for a summative usability test. I will also provide some tricks and tips to help you get the most out of your test participants and testing.
-
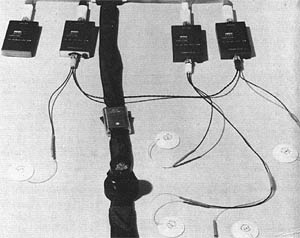
Apollo program engineers collected vital biomedical data from the astronauts using biomedical telemetry to monitor crew health.
-
Watch the most viewed StarFish Medical videos of 2018 to learn about medical device design, development, regulations, and manufacture.
-
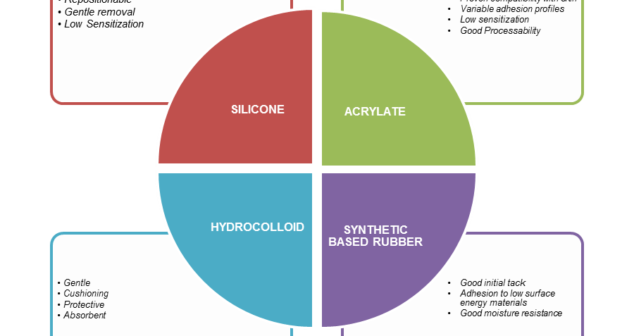
Successful wearable medical devices and adhesive selection require solid understanding of the basics of adhesives along with Do's and Don'ts.