Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Every MedTech startup begins with a hypothesis, an idea that could transform patient outcomes, simplify delivery of care, or improve how clinicians diagnose and treat patients.
-
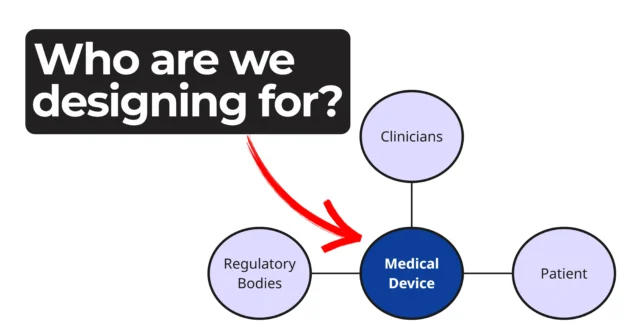
Every phase of a device’s life cycle involves different people with distinct needs—from clinicians and patients to service technicians and regulatory bodies.
-

Your team is ready for design validation. The prototype performs well, test plans are in motion, and everything points to a smooth handoff to manufacturing. Then your partner calls with bad news: they can’t build the device as designed.
-

You’ve cleared the toughest engineering hurdles and proven your design works. Then, just as you prepare to scale, your contract manufacturer turns you down.
-
In Medtech, a successful exit isn’t just about having an innovative device, it’s about building a business that potential buyers and investors can clearly see a future in.
-

Being ready to build is not the same as being ready to scale. Successful market introduction requires manufacturing readiness that evolves alongside market readiness.
-

Ariana Wilson and Mark Drlik explore how teams can reduce the device development timeline without compromising quality or compliance.
-
Understanding medical device classifications is critical for compliance, risk management, and time-to-market success. Whether you’re designing a wearable sensor or a life-sustaining implant, knowing how your device fits into FDA Class 1, 2, or 3 categories is essential.
-

Choosing the right design and development partner is one of the most critical decisions in bringing a medical device to market.