Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Tips, considerations, and techniques to increase IVD Regulatory Success that is essential for many Medical Devices.
-

Reduce costs by reusing IVD Verification Test equipment for verification of optical components as part of manufacturing quality control.
-

Many (IVDs) employ optical techniques to sense. How to develop low cost IVDs suitable for Point of Care use – optical sensing.
-
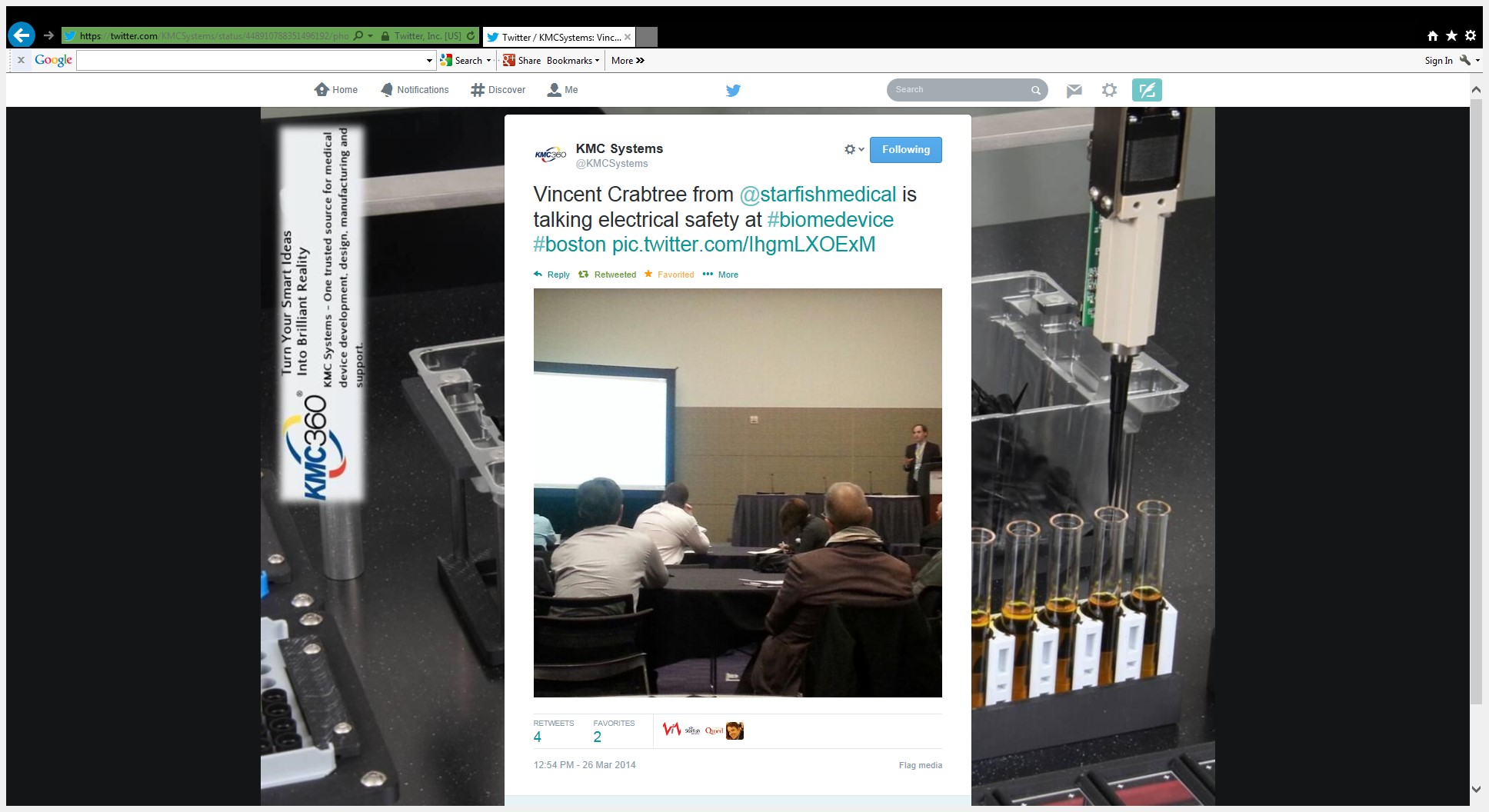
Highlights from Day 1 of Learning Lab event at Boston BIOMEDevice on electro-medical device basic safety and achieving compliance with 60601.
-

Proven Process for Medical Device Success– Pathfinder Process: Regulatory, Human Factors and Workflow, Technology Assessment, Design for Manufacture.
-
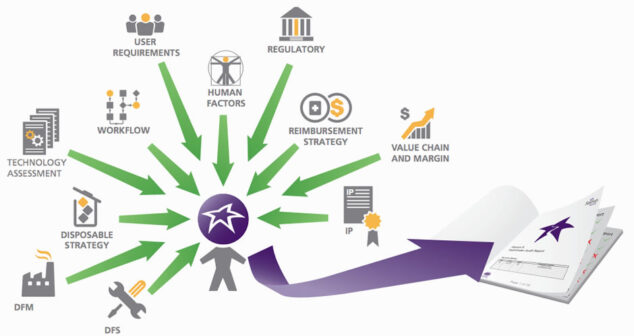
Medical Device Pathfinder process for Intellectual Property, Consumables Strategy, Value Chain and Margin, and Reimbursement.
-

The best way to avoid waste is to consider medical device regulatory requirements at a project outset.