Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Medtech founders operate with more constraints than most sectors. You are responsible for deep technical problem solving, high-stakes decisions, regulatory navigation, investor conversations, and a constant stream of operational tasks.
-

When Ariana Wilson and Mark Drlik take apart a common appliance, they uncover engineering principles that connect directly to medtech.
-

When reviewing evidence for a medical device, a single citation can shape an entire submission. In this Bio Break episode, Nick shares a biofilm referencing lesson that has stayed with him since the early 2000s.
-
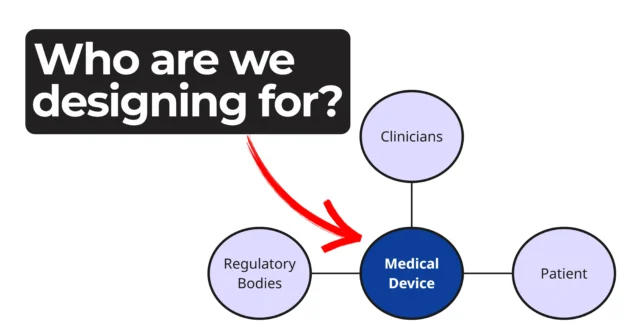
Every phase of a device’s life cycle involves different people with distinct needs—from clinicians and patients to service technicians and regulatory bodies.
-

Nick Allan and Nigel Syrotuck explain how a fluorescent protein assay helps engineers measure contamination and cleaning performance in medical devices.
-

Ariana Wilson and Mark Drlik explore how teams can reduce the device development timeline without compromising quality or compliance.
-

Nick Allan and Nigel Syrotuck explore a creative approach to visualizing cleaning validation using a fluorescent soil load.
-

Nick Allan joins Nigel Syrotuck to explore how anaerobic sample collection works and why it’s vital for studying bacteria that cannot survive in oxygen.
-
Understanding medical device classifications is critical for compliance, risk management, and time-to-market success. Whether you’re designing a wearable sensor or a life-sustaining implant, knowing how your device fits into FDA Class 1, 2, or 3 categories is essential.