Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Predetermined Change Control Plans (PCCPs) Draft Guidance with Implications for Medical Device Manufacturers and regulatory framework
-

Crafting powerful business presentation materials requires a subtle balance between visual appeal and clearly written content.
-
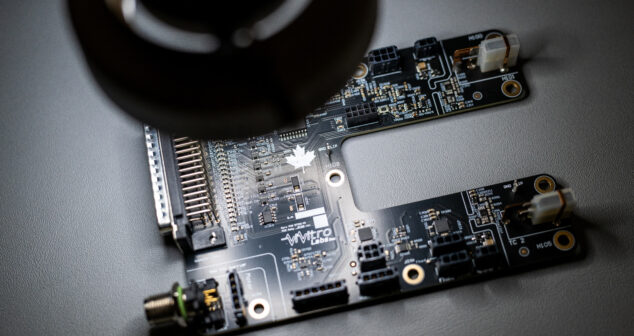
Continuous Integration (CI) firmware will help medical device software developers show regulators that their code is of high quality.
-

Kevin Chaplin, CEO of Kardium Inc., shares the remarkable story of Kardium’s rise as a pioneer in atrial fibrillation therapy.
-

“From Scrappy Startup to Trailblazer: How Redlen is Revolutionizing CT Therapy” is now available as an on demand webinar.
-

In this episode of Bio Break, Nick and Joris dive into the fascinating realm of biosensors, showcasing how nature’s biological processes inspire groundbreaking innovations in medical device technology. From jellyfish to fireflies, the natural world has provided invaluable tools that are transforming diagnostics and research.
-

Learn the entrepreneurial experience of launching a new product into a space dominated by a formidable competitor.
-

On Demand recording of Automating the pre-commercial exit as a strategy for high-value startups presented by Amr Salahieh, serial entrepreneur and founder of Sadra Medical and Shifamed, at Medical Device Playbook Newport Beach 2024 is now available for on demand viewing.
-

Drawing on their extensive experience in executive roles and high-tech M&A, the speakers delivered an engaging discussion tailored for MedTech founders and executives.