Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Key variables in rapid silicone prototyping are material selection and silicone moulding strategy. Approaches and key points for mould design.
-

Five points to consider. from the dot-com era to make our medical device digital health strategy bubble proof.
-

4 ways (with related principles) to make your medical device jig design process more effective. Jigs help us conduct tests and collect data.
-

Adaptive studies can be especially useful in the pivotal stage if there are uncertainties about one or two aspects of the study.
-

Medical device designers and developers need to address stress, fear, and embarrassment a patient might feel. as part of emotional design.
-
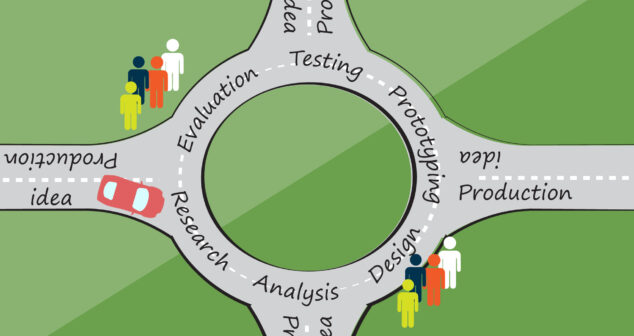
The UX design process for medical device research places emphasis on safety and documentation using IEC 62366-1 and IEC 62366-2
-

Digital Health communication technologies considerations for medical devices rule number one: Understand the information needs.
-

Digital Health communication technology starts the concept that data from a device will need a consumption platform to receive it.
-
Digital Health roadmaps are a great tool for partnership alignment, use cases, and evaluating the value of integrated support services.