Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Review of cost-associated parameters you should consider before contracting with an injection moulding company for medical devices.
-

Prototyping medical devices should not be skipped due to cost. The look, feel, and perception of the product can be critical to its success.
-
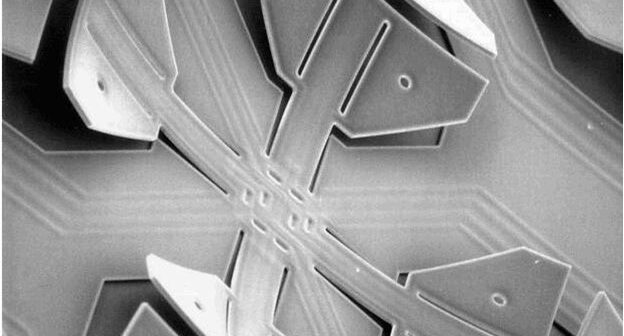
Microelectromechanical Systems (MEMS) are tiny computer chips with moving parts. Wearables are information accessibility for medical devices.
-

Process to choose the right medical device colours for those without a design background or colour training.
-
Colour impact of medical device design can increase usability and influence user/patient emotional response diminishing negative feelings.
-

Colour in medical device design including trends and branding, functionality, emotional connection and a process to help determine colours.
-

Comparison of DC motors and stepper motors when designing the motion control systems of Medical Devices. Advantages, Disadvantages, Tips.
-
Digital Health & medical device regulatory and technical implications: HIPAA, Mobile Medical App (MMA), Medical Device Data System (MDDS), and app stores.
-

Garbage In, Garbage out…