Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Whether you're in the early stages of a new project or refining a product concept, this episode of Bio Break delves into the foundational importance of a well-defined Target Product Profile (TPP) in medical device development. It's packed with practical advice and expert insights to set you on the path to success.
-

In this episode of Bio Break, StarFish Medical experts Joris and Nick explore the groundbreaking FDA clearance of the first over-the-counter continuous blood glucose monitoring system. This exciting development marks a significant milestone in the world of diagnostics and opens new possibilities for patient-centered healthcare.
-

SBOM Analysis and Value covers the FDA “Cybersecurity in Medical Devices: Quality System Considerations and Content of Premarket Submissions guidance, role of Software Bills of Materials (SBOMs), and how to create them.
-

QNX Medical Device Bootscreen tips for the popular micro-kernel OS designed for embedded systems and safety critical hardware.
-

2024 FDA guidance on medical device cybersecurity covering risk management, design controls and software validation is explained in this article. Cybersecurity, Risk Management, Secure Product Development Framework (SPDF) are also covered.
-
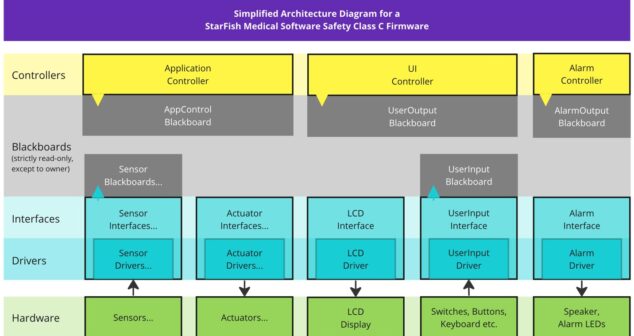
How to develop Class C Firmware for medical devices and implement Segregation in compliance with the IEC 62304 Standard.
-
Risks, potential roles, and tips for using Large Language Models (LLM) or (AI) in medical devices effectively and responsibly. Role of AI in medical devices: In the medical field, generative language models, colloquially known as “AI” or "LLM" must be used responsibly to enhance the skills of clinicians or improve patient experience without exposing either to increased risk.
-

Medical Device Design for Testability during development explores the pros and cons of its application throughout the entire design process design concern throughout the whole design process, ensuring that all parts of the product can be both manipulated and monitored allowing for thorough testing.
-
Three common elements can be observed in biomedical product development projects that are doomed to failure. They are the Three “P’s” – Platform, Point of Care, and Program Manager.