Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Highlights of July and August 2014 medical device FDA guidance documents that reduce regulatory burden without compromising patient safety.
-

Techniques for IVD algorithm development. IVD algorithm is often required to convert electronic sensor data into a clinical parameter.
-

Most people average 5 different careers. It’s a great way to rediscover and rebrand yourself. Medical Device career path of QA specialist.
-

Small technology is more available and more socially accepted changing the way engineers approach problems in aerospace and medical devices.
-
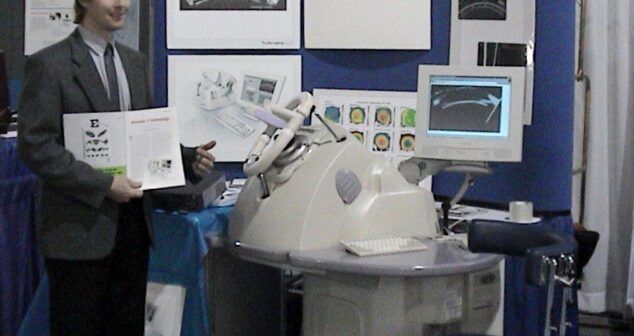
Ultrasound Primer: Diagnostic imaging device, used for analyzing body fluids, treating kidney stones, enhancing drug delivery, and managing pain.
-

Entrepreneurial company uses StarFish Medical device process and materials to improve their business and medical device success.
-

Tips, considerations, and techniques to increase IVD Regulatory Success that is essential for many Medical Devices.
-

Will consumer electronics engulf the medical device development community, making rigorous testing and risk mitigation processes obsolete?
-

MDSAP enables Medical Device companies to save in Registrar’s fees, eliminate duplication of inspections and gain faster access to markets.