Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Nick Allan and Nigel Syrotuck share their end-of-summer reading list, featuring FDA regulatory books and PCR memoirs. From navigating regulatory hurdles to celebrating groundbreaking discoveries, their choices show how science reading can be both educational and entertaining.
-

Project managers are on the front lines of rising complexity in medical device development. They sit at the intersection of vendor timelines, regulatory constraints, and engineering realities.
-

As a software engineer with experience in both web development and medical system software engineering, I’ve worked on projects ranging from consumer-facing web applications to medical device graphical user interfaces (GUIs).
-

Many developers have tried using AI to generate code, often called “Vibe Coding”. Sometimes, the results are nothing short of amazing. Other times, the results are mixed, or worse.
-

Even the best-designed devices, prepared with careful simulations and usability studies, can behave very differently when used in actual clinical or emergency situations.
-

In this Before the Build episode, Eric Olson and Paul Charlebois dive into the importance of organ transplant logistics when designing effective medical devices.
-
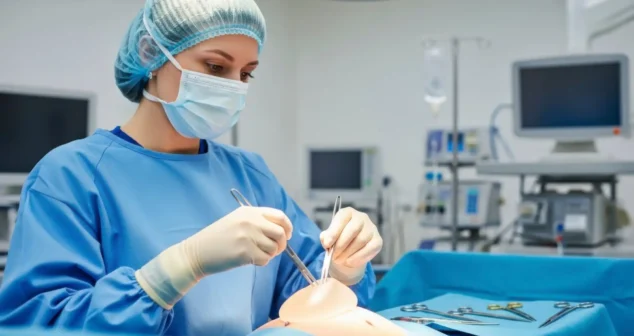
The simplest and least expensive way to train users of medical devices is to ask them study the Instructions for Use (IFU) beforehand.
-

In the complex world of medical device development, creating effective prototypes is more than just a creative exercise, it’s a regulatory necessity and a strategic imperative.
-

User Experience (UX), Human Factors (HF), and Industrial Design (ID) each have a major impact on the success of new medical devices. Their influence is especially important during product definition and early phases of device development.