Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Overview of tools for Root Cause Analysis is an important tool in medical device development and manufacturing.
-

Nick and Joris tackle a topic that’s more relevant than ever—raising funds for medical device development in today’s challenging financial landscape. With economic headwinds and cautious investors, startups and even established organizations face significant hurdles in securing funding. But there are ways to mitigate these challenges, and Joris shares strategic insights to align product development milestones with fundraising cycles.
-

Whether you're in the early stages of a new project or refining a product concept, this episode of Bio Break delves into the foundational importance of a well-defined Target Product Profile (TPP) in medical device development. It's packed with practical advice and expert insights to set you on the path to success.
-

Medical Device Product Development Tips from experts covering engineering, design and development in Phase One of Development.
-

Company values that StarFish employees use most to develop better medical devices include looking deeper, cutting to the chase and getting better.
-
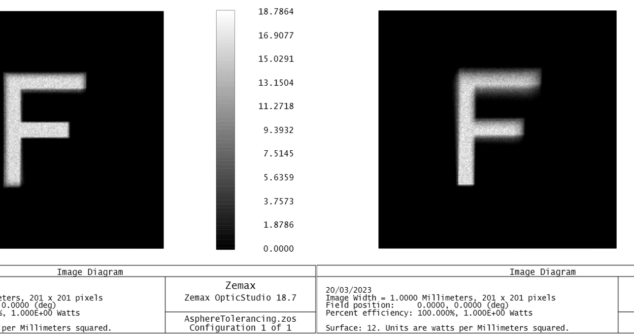
Overview of tolerancing simulation for medical device optical systems in Zemax OpticStudio’s sequential mode.
-

Top medical device project management lessons learned from more than a quarter century of product development experience.
-

Guide to run a lean and agile medical device product development programs using with four considerations and key milestones.
-
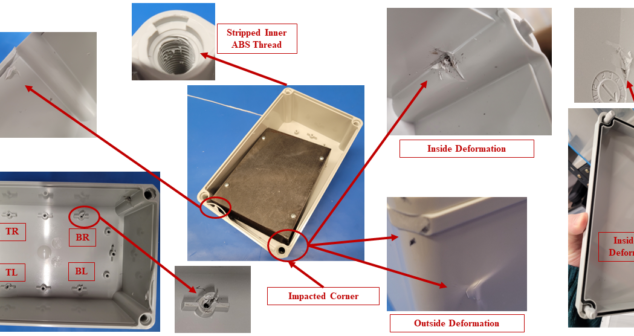
In silico medical device drop testing offers scalability, fast-tracking of iterations, and the ability to review hard-to-detect failure modes.