Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Strategies to maximize outcomes for interactions during regulatory pre-submission meetings using 4 simple, but effective steps.
-

FDA cybersecurity requirements for medical devices which are considered “cyber devices”, including US government definition.
-
Overview of eSTAR, a joint Health Canada and FDA program streamlining medical device submissions with info on how to apply for the program.
-

StarFish engineers, QA/RA, project management, and manufacturing experts offer medical device commercialization resolutions for 2023..
-

VR Design Tools – how does VR stack up to conventional methods in sketching and CAD? Discover areas in the design process where it excels.
-
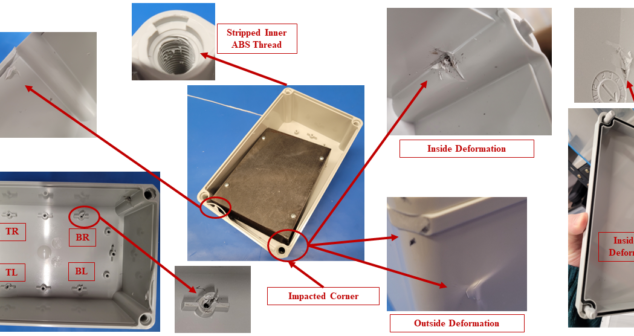
In silico medical device drop testing offers scalability, fast-tracking of iterations, and the ability to review hard-to-detect failure modes.
-

Three concepts to improve teaming in medical device project management group are Handshakes, 5 Stages, and 6 Hats.
-

Favorite books in 2022 recommended by StarFish Medical bloggers and avid readers who contributed a list of books they enjoyed most in 2022.
-

We share our newest and most interesting blog posts in a monthly newsletter. Inevitably some articles prove more popular than others, so…