Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

StarFish employees share their bucket list vacation activities, including outdoor activities, family, travel, community, education.
-

Six principles to ensure your medical device project plan is useful and successful from a seasoned project manager.
-
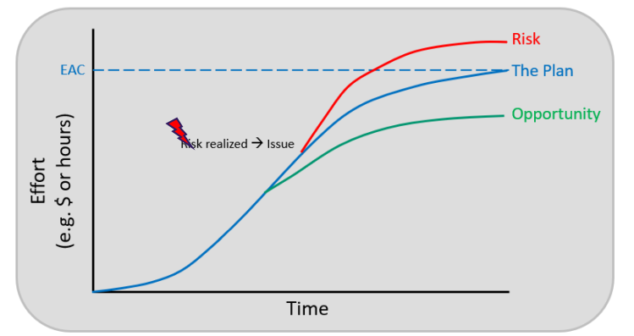
Proven approach to medical device development risk management using an every day concept using plan, risks, opportunities, and actions.
-
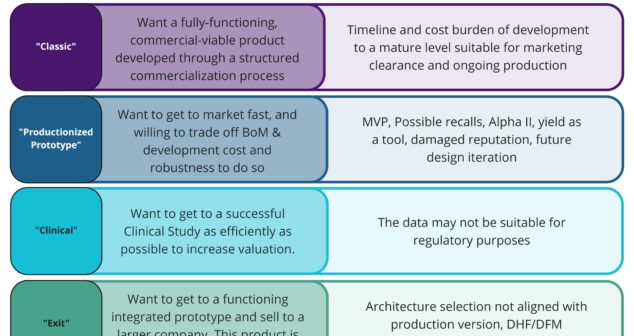
Six common medical device product development destinations and overview of a High Level Program Planning (HLPP) approach.
-

Learn about the FDA's 2023 guidance on premarket submissions for device software functions and its impact on the medical device industry.
-

The FDA released a new Cybersecurity draft guidance on April 2022. It is intended to replace the current final guidance from 2014 which is well overdue an update. The draft guidance significantly expands requirements for cybersecurity activities and documentation for medical devices. The intention is to align medical device development with current best practices from other industries. This white paper reviews these new requirements and considers their impact on medical device developments.
-

Writing and implementing standard operating procedures (SOPs) for the manufacture of medical devices is required by ISO 13485, FDA, and other regulatory bodies. An SOP is a set of written instructions that documents a routine or repetitive activity that is followed by employees in an organization. The development and use of SOPs are integral parts of a successful quality system. They provide directions to perform a job properly and consistently to achieve predetermined specifications and quality end results. SOPs address all requirements to complete the job or process safely and effectively.
-

Systems engineering can enable creativity and nurture a collaborative development culture for Medical Device Creative Collaborations.
-
4 insidious hidden costs in medical device development ranging from iterative processes to best practices, and best practices to avoid them.