Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
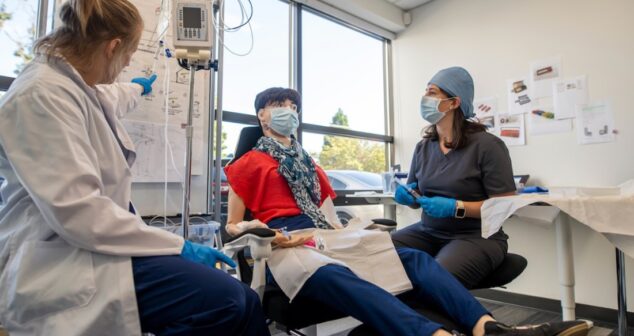
Medical Device User Testing Tips to get the most out of user testing, a critical part of the medical device design process.
-

Cardboard Vs. 3D Rapid Prototyping compares low fidelity cardboard mock-ups with high-fidelity 3D printing models for human factors engineers.
-

How extended reality (XR) environments like VR, MR and AR will revolutionize medical device design using a variety of real examples.
-
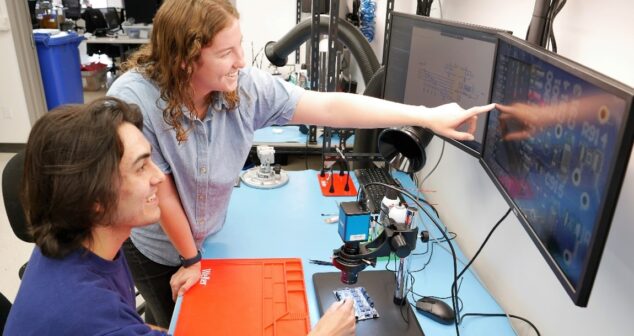
Electrical Engineering Co-op students share their experiences and provide answers to EE Co-op FAQ (Frequently Asked Questions).
-
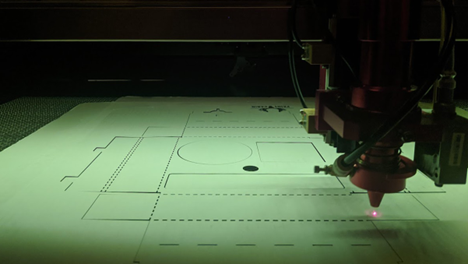
Tips for using laser cutting as a go to tool in medical device design include software, applications and useful examples.
-

Summary of UK Medical Device Brexit Implications including high-level key points of the MHRA post-Brexit implementation plan.
-

FDA DHT Clinical Investigations examines the latest FDA draft guidance on Digital Health Technology for remote data acquisition in clinical investigations.
-
Analysis of QMSR ISO 13485:2016 Alignment in FDA proposed changes to Quality Management System Regulation for alignment with ISO 13485:2016.
-

Medical device cyber security: 2022 Update includes review of regulations and implications in an ever-evolving landscape of vulnerabilities.