Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Define medical device ID strategy early, A strategy document can quickly align a large interdisciplinary team on a product's design direction.
-

Any medical device connected to a hospital network or the cloud may be exposed to some form of medical device cyber attack.
-

Differences noticed between design in school and in real world medical device design during my internship at StarFish Medical.
-
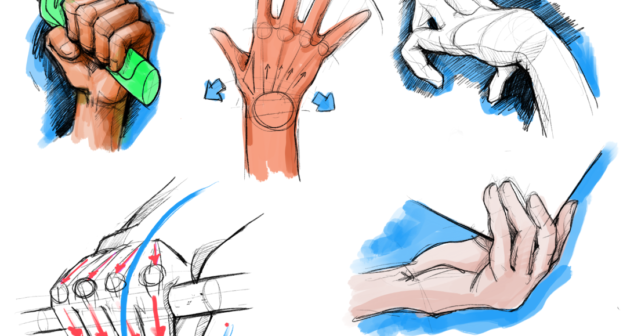
Medical device sketching is an effective tool to help clients visualize how their ideas might look like as a fully realized product.
-
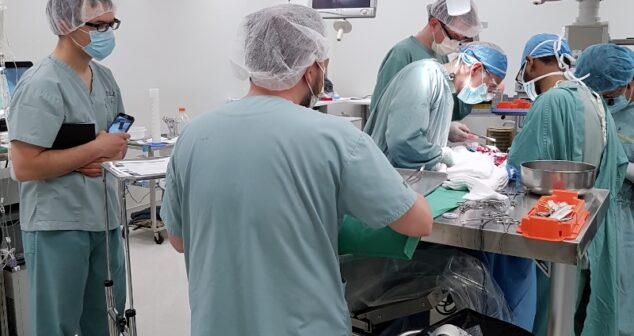
Procedural observations of medical procedures are invaluable and inform designs with perspective of users, procedural workflow and intended use environment.
-

3 Medical UX design differences between the graphical user interfaces (GUI) of digital displays in medical devices and consumer products.
-
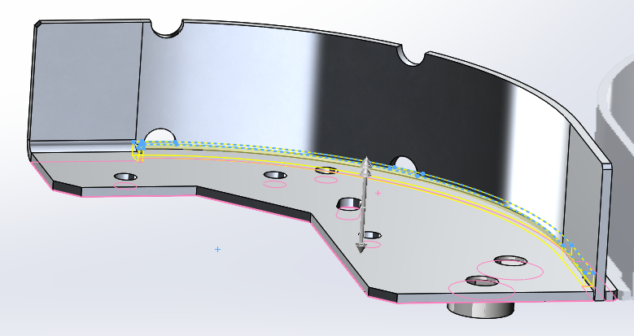
How to prototype medical device parts in 4 steps by using SLS printing to replace traditional sheet metal prototype parts.
-

Comparison of the biggest unknown between IEC 62366:2007 and IEC 62366 -1:2015– Fig. 5.10 User Interface of Unknown Provenance (UOUP).
-

Simulated Fluids help avoid high costs of animal trials and exposure risks to infectious biological for early stage medical device design.