Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
Many clients now request their devices to look and feel like Apple products. But achieving that level of simplicity and elegance is not as easy as it seems.
-

You have a great MedTech innovation idea and are trying to decide whether to build a team to commercialize a medical device…
-

The costs of early-stage medical device development in North America and Europe continue to rise. Increasing technical complexity and the compounding costs of nonclinical and clinical evaluations are driving this trend.
-

Today, 85% of the top 50 healthcare companies use Computational Modeling and Simulation (CM&S) to develop their products and processes. Whether it’s refining overall device parameters or optimizing critical requirements, engineering simulations help reduce development timelines and enhance design exploration.
-

Paul Charlebois and Eric Olson explore how contextual inquiry in MedTech drives smarter product design. By observing how users interact with devices in real settings, product teams can gather early insights that shape usability, adoption, and safety—long before development begins.
-
Ariana Wilson and Mark Drlik dive into how the FDA is adopting artificial intelligence to modernize its regulatory processes. With a new chief AI officer in place and rumors of collaboration with OpenAI, the agency is taking major steps to automate review workflows and improve efficiency.
-

In a sophisticated world of ever increasing complexity, we need our tools to evolve alongside us and assist in complex decision making, allowing us to understand the consequences of choices ahead. Computational Modelling and Simulation (CM&S) is emerging as an essential tool in building evidence for medical device development.
-
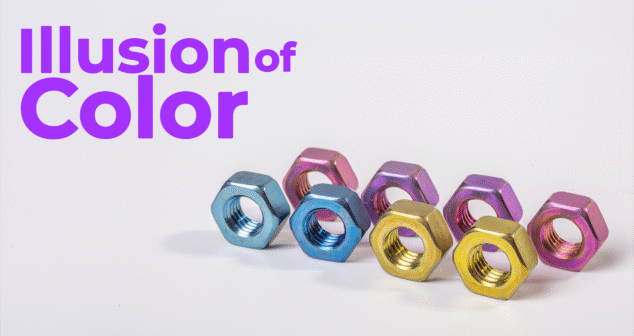
We explore the fascinating intersection of materials science and usability in medical device development. Mark Drlik and Ariana Wilson discuss how anodized titanium produces vibrant color without dyes, and how this visual property supports surgical safety, device differentiation, and biocompatibility.
-
Nick and Joris break down what a DHF is, why it's required, and how it plays a vital role throughout the development lifecycle.