Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

A key aspect of successful project management is sticking to the budget. 3 budget monitoring strategies to help predict the unpredictable.
-

What’s the best way to motivate medical device teams? What used to work may not apply any more.
-

Risk management is a key element in your innovation process and an excellent skill. Here are five pro tips to managing innovation risk
-

Help guide client and team to achieve project success by considering potential pitfalls and mitigating project risks.
-
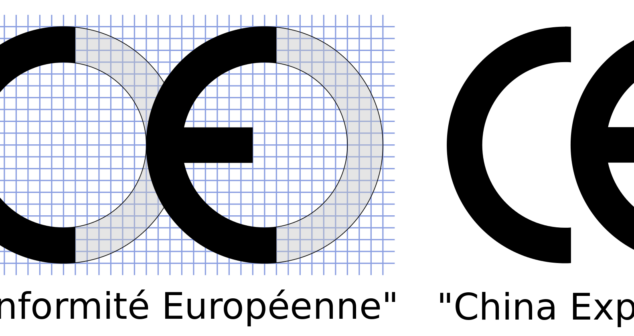
CE vs China Export Mark Explained – Discover meanings, regulations and visual layout of the two marks to avoid costly mistakes.
-
Advice to the novice PCB designer for developing and designing medical device PCB prototypes. Advanced designer feedback is welcomed!
-
Compare 3D printing prototyping merits for three common processes with handy chart and process descriptions.
-

Medical device miniaturization approaches, options and trade-offs when miniaturizing a device’s electronics.
-
The easiest way to decipher comfort when designing a medical device is a combination of irritate testing and asking exact questions.