Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

One of the key requirements for ISO 60601-1 is the protection against water ingress in Medical Devices. Three areas to watch are discussed in this article..
-

Realities and tips for designers and developers managing medical device software projects from project management professional..
-
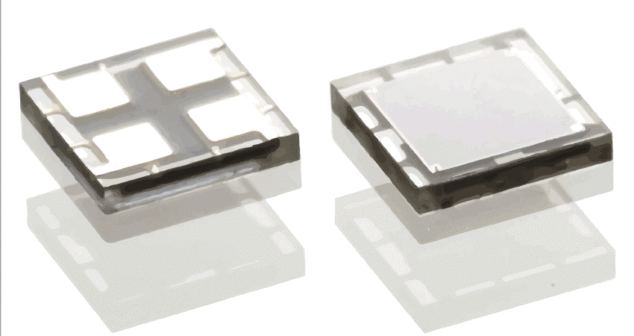
Silicon photomultipliers are an exciting technology enabling applications to be implemented in small, low-cost, low-power form factors.
-
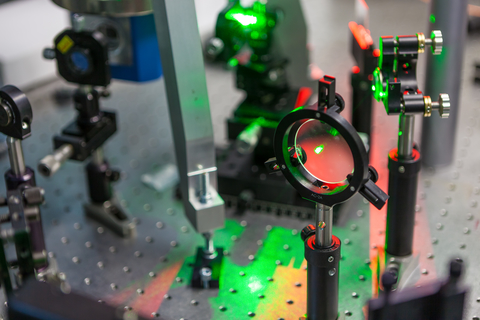
Optics labs best practices to help create an optics lab environment that is clean, organized, efficient, and produces great data.
-

StarFish employees share their summer plans and favorite warm weather activities to celebrate the summer solstice. There is definitely a theme.
-

There are a lot of things to consider when creating a summative usability test. The first time can be overwhelming. What scenarios do I test? How do I identify a critical task? How many participants do I need? Do I need to test the training? In this eGuide I will help you avoid common mistakes, like using a formative test structure for a summative usability test. I will also provide some tricks and tips to help you get the most out of your test participants and testing.
-

Insights and observations that spark medtech innovation are brainstormed by team of medical device professionals at StarFish Medical.
-

Prototype a medical device on a budget using low-cost tips and methods employed in New Product Introduction (NPI) work.
-

Medical Device Playbook was conceived to inspire entrepreneurship and connect the many players. Here are commercialization Insights from attending the event.