Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

7 interesting outcomes of the 2019 Health Canada Action Plan on Medical Devices initiatives and alignment with international regulators.
-
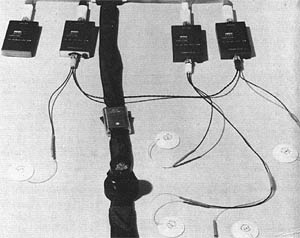
Apollo program engineers collected vital biomedical data from the astronauts using biomedical telemetry to monitor crew health.
-
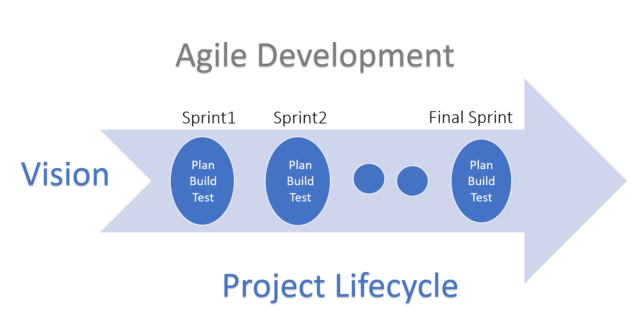
Agile methodology in Medical Devices Product Development methodology is supported by an FDA guidance and IEC standards.
-

Article offers 10 Ultra Low Power Embedded Design tips to meet growing demand for medical devices that do more with less power.
-
Co-op job tips on how to increase chances of landing an interview and what to expect during the StarFish interview process.
-
Five tips and tricks for ElectroMagnetic Compatibility (EMC) testing, a key part of medical and other device development.
-

9 medical device commercialization resolutions recommended by our team of engineers, designers, QA/RA, and manufacturing professionals.
-
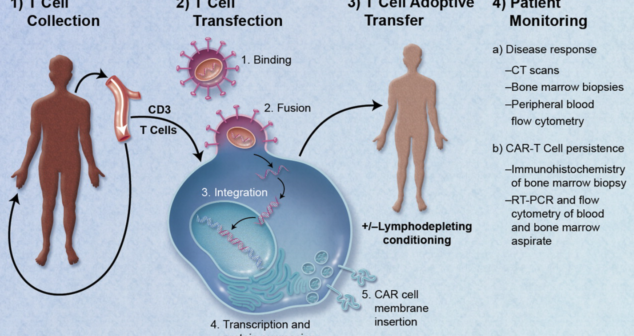
Regenerative Medicine (RM) therapeutic medical devices transform healthcare. The role of Medical Devices in the RM industry is changing.
-

Two things you should consider in order to be successful when you are developing a Regenerative Medicine Medical Device Design