Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
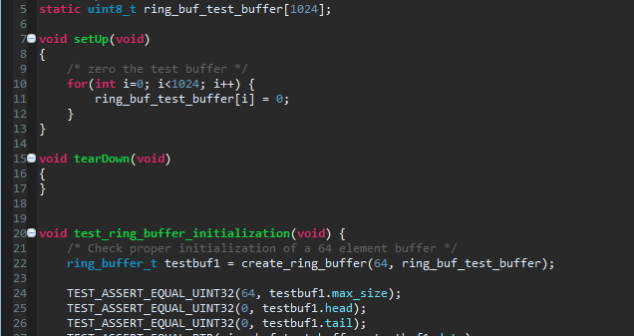
Debunks three common unit testing myths and shares author's experience using an automated tool when designing medical device firmware.
-

Interesting finding in core clock speed for Raspberry Pi 3 Compute module (CM) and the default Raspbian image. Impact for medical device.
-

Dr. Joe Burkett, an emergency medicine physician shares his Medtech entrepreneur journey as Founder & CEO of Vaid Medical Devices.
-

CPU Modules can be incorporated onto a custom PCB in a medical device or IVD providing a powerful CPU on a product specific PCB without designing the CPU onto their board.
-
Under-estimate the time to construct and debug prototypes? Tips on saving time (money) during the build and debugging of a prototype.
-

ISO/IEC 17025:2017 includes many changes. Three points to keep in mind: more options, involvement of risk, updates in current technology.
-
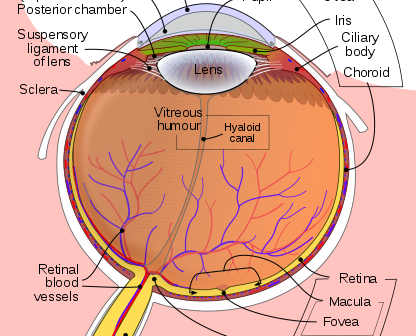
Five eye diseases that account for the majority of issues related to blindness or poor vision. Diagnostics and treatment of these diseases are often performed with precision opto-mechanical medical devices that continue to increase in reliability and accuracy and decrease in price every year.
-
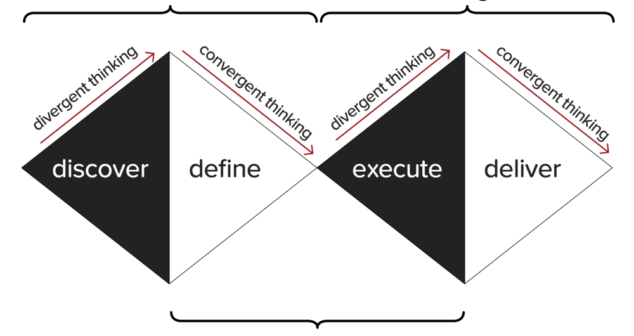
Kepner-Tregoe’s original Decision Matrix overview with tweaks to make it broader and more robust.
-

Five things project managers can do to achieve great results and empower team members to perform at their best.