Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Proven ways to manage technical teams with the goal of everyone wins. The result is a happy, collaborative team and satisfied client.
-
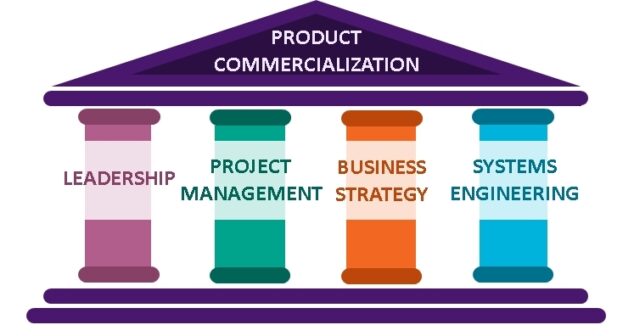
Project management group discusses the structure which enables medtech innovation on budget and on time using four pillars supporting technology commercialization.
-
Is Anodized Aluminium Biocompatible? Learn whether anodized aluminum meets ISO-10993 (a standard for biocompatibility).
-
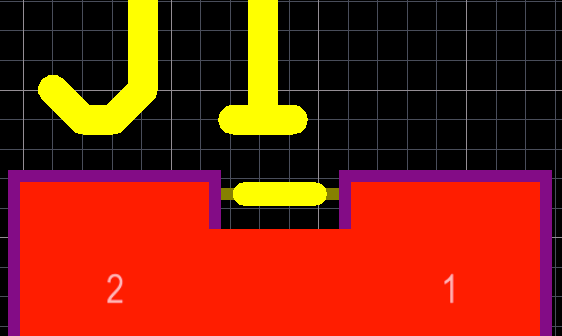
PCB design tips to design your board for ease of assembly, testing, and modification every time you build them.
-
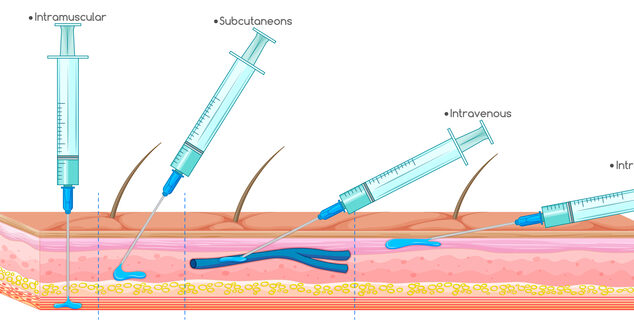
Injection devices like needle-tipped plastic syringes haven’t changed much in the 60+ years, but new technologies have significant advantages.
-

Managing evolving requirements is a core competency for medical device technical specifications and understanding health care end users.
-

Open Source Software Medical Devices: Using OSS libraries within a Software System of a Medical Device or In Vitro Diagnostic Device.
-

FDA Guidance on Additive Manufacturing (AM) – offers new ways to integrate parts not only for prototypes, but for final products.
-

Why design control is required for medical devices and is superfluous in the consumer goods design with advantages and disadvantages.