Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-
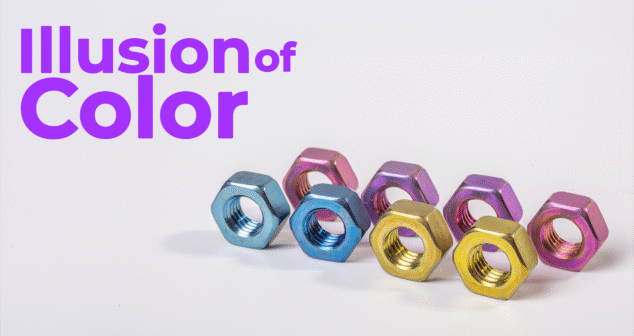
We explore the fascinating intersection of materials science and usability in medical device development. Mark Drlik and Ariana Wilson discuss how anodized titanium produces vibrant color without dyes, and how this visual property supports surgical safety, device differentiation, and biocompatibility.
-
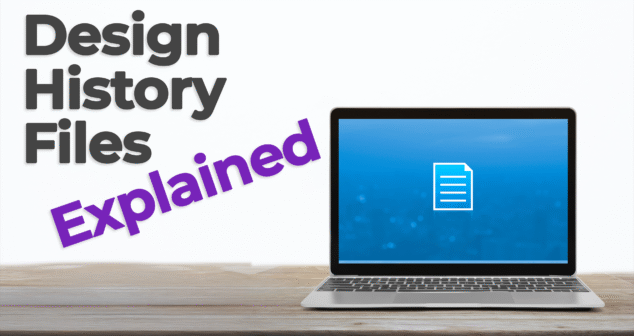
Nick and Joris break down what a DHF is, why it's required, and how it plays a vital role throughout the development lifecycle.
-
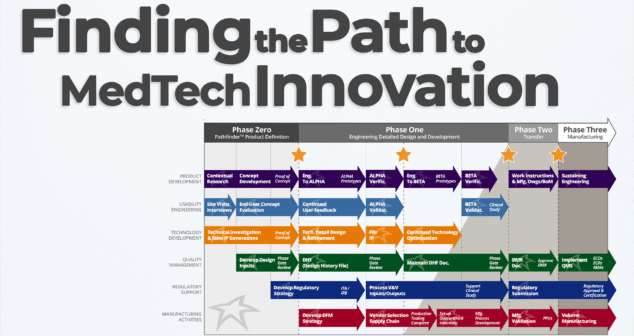
Nick and Joris explore one of the most dynamic early-phase services at StarFish Medical: the Pathfinder Program. If you're a medtech innovator with a promising concept or prototype, Pathfinder helps you identify the right path forward—before you invest millions in development.
-
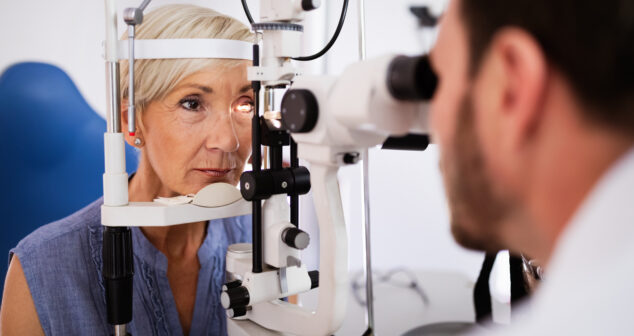
Optics Physicist and Engineer share approaches to performing pre-screen Ophthalmic Instrument Safety Assessment testing in-house.
-
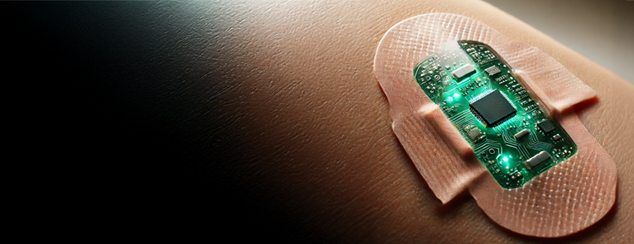
With the recent developments and seemingly ubiquitous nature of real time glucose monitoring and availability of smart wearable tech, the development of a theranostic band-aid seems inevitable. But how practical would this be? Is there a Theranostic wound dressings market?
-
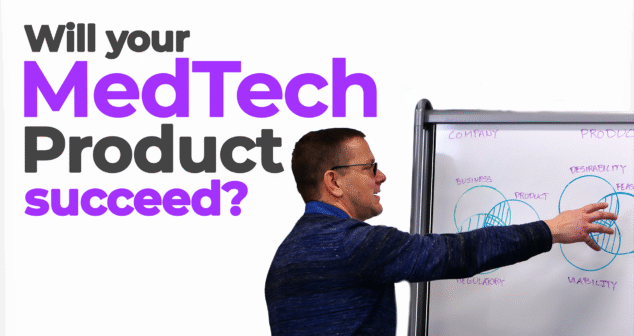
Ariana Wilson and Mark Drlik break down a powerful visual framework for understanding what makes a medtech product, and the company behind it, truly successful. Using a triple Venn diagram, Mark explains how strategic alignment across feasibility, viability, and desirability can drive better product outcomes and business success in the medical device industry.
-

What are the real costs of developing a medical device? In this episode of Bio Break, Nick and Joris dive into one of the most frequently asked questions they hear from clients: How much does it cost to develop a medical device?
-

We all know medical devices have labels, but how often do we consider their purpose and the effort required to ensure they provide the right information? Device labelling serves as the interface between the manufacturer, the user, and regulatory bodies. (Note that being from Canada, we spell labelling with two Ls.)
-

Sterilization is a critical process in the medical device industry as it provides a reliable way to ensure that devices are free from harmful microorganisms when they are used on patients. This blog talks about the categories of sterilization currently used on medical devices in manufacturing settings. It also addresses concerns surrounding the use of ethylene oxide (EtO), an indispensable method for sterilizing heat and moisture sensitive devices.