Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

We all know medical devices have labels, but how often do we consider their purpose and the effort required to ensure they provide the right information? Device labelling serves as the interface between the manufacturer, the user, and regulatory bodies. (Note that being from Canada, we spell labelling with two Ls.)
-

Common mistakes in medical device projects can create roadblocks that, if left unchecked, can snowball into costly setbacks.
-

ASTM D4169 Options for this standard test method of performance testing shipping containers and packaging systems are explored.
-
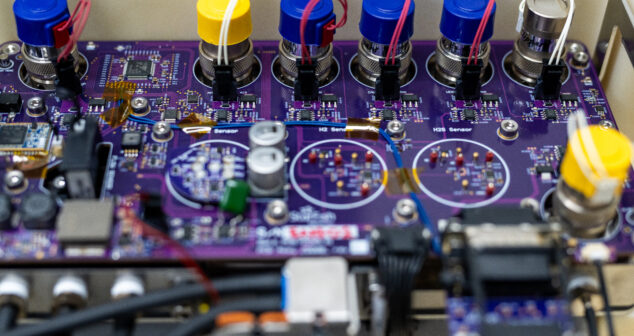
Getting a PCB (Printed Circuit Board) for a medical device right the first time is almost impossible. Datasheets can be misleading, or assumptions and architectures change. As a result, modifications are almost inevitable. Sometimes the modification is as simple as swapping resistors or adding capacitors. Other times it involves tacking on new circuits you had no idea you needed.
-

This blog explores key highlights of the Chemical Analysis for Biocompatibility Assessment of Medical Devices draft guidance, focusing on its scope, testing methodologies, and recommendations for reporting. In September 2024, the U.S. Food and Drug Administration (FDA) issued a draft guidance titled Chemical Analysis for Biocompatibility Assessment of Medical Devices.
-

Nick and Joris explore the fascinating world of repurposing existing medical device technologies for new market sectors. As engineers and innovators, we often focus on creating brand-new solutions, but what about leveraging tried-and-true technologies to expand into untapped markets? This strategy not only opens doors to new revenue streams but also maximizes the potential of existing innovations.
-

Fluid-structure interaction (FSI) modeling is transforming the medical device industry by simulating complex dynamics between biological fluids and medical devices. In a field where safety and precision are paramount, FSI modeling offers engineers and researchers a powerful tool to design, test, and optimize devices in a virtual environment before physical prototypes are created or clinical trials are conducted.
-

Cleanroom best practices are crucial to maintain a contamination-free environment, especially in industries like pharmaceuticals, semiconductors, biotechnology, healthcare, and medical devices. Here are some essential guidelines for ensuring your cleanroom is indeed clean.
-

Medical device design transfer is a critical phase in the development process, marking the transitory phase from the design and development stage to manufacturing. This phase ensures that the medical device continues to meet its safety, effectiveness, and regulatory compliance once production begins. It is the final phase in the development process and sometimes overlooks or underestimates the amount of time and effort required to ensure that final manufactured devices meet requirements.