Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Medical Device UX Design is differentiates companies and products from the market. Here are three common mistakes to avoid.
-

How to determine Project Management potential and technical expertise when considering medical device service providers.
-

How good ID impacts medical devices from engineers, project managers, manufacturing, and QA ID and UX professionals
-
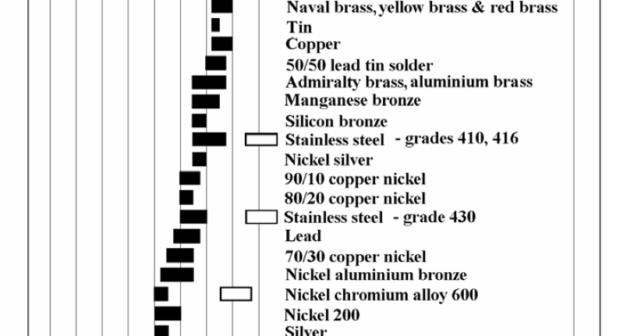
Causes for stainless steel rusting include inter-granular corrosion, microbial staining. Why stainless steel rusts and how to prevent it.
-

Christian McMechan and Heidi Giesbrecht share their experiences and thoughts on working at StarFish Medical with Scott Phillips.
-

Surprised medical devices have poorly thought-out asynchronous serial protocols to behave safely and correctly during normal operation?
-

Engineers, regulatory advisors, and patent filing teams should review the MAUDE database as part of development and due diligence process.
-

Doug Evans, CEO Lungpacer Medical, former COO & board member of Kensey Nash Corp., discusses medtech start-ups, scaling, and creating block busters.
-

Joel Weinstein is the real deal medical device serial entrepreneur. Joel spoke at many Medical Device Playbooks starting with Toronto 2016.