Resource Centre
Discover a wealth of knowledge and insights from the experts at StarFish Medical. Our Resource Centre offers product development tips, reviews of new and cutting-edge technologies, and in-depth articles on regulatory updates and compliance in medical device development.
-

Rules for Medical Device Entrepreneurs from Paul Geyer captured during medical device executive panel discussion at #MDPLAYBOOK.
-

Interview with Larry Spears, Former Deputy Director FDA/CDRH Office of Compliance and FDA Investigations on prep for regulatory submissions.
-
Two ways outer space will impact medical devices: 1. Access to Medical Information and 2. Medical Information’s access to you.
-

Medical Device Development Outsource Selection using The Checklist Manifesto by Atul Gawande, MD as basis for five checkpoints.
-

Health Canada has announced online publication of Regulatory Decision Summaries (RDS) and a list of decisions under review.
-
RoHS is still a hot topic. Background on RoHS compliance with 11 parts and processes to watch out for during the design stage.
-
Digital Health & medical device regulatory and technical implications: HIPAA, Mobile Medical App (MMA), Medical Device Data System (MDDS), and app stores.
-

Digital Health Advantages and Disadvantages describes numerous advantages and motivations for medical devices to engage with Digital Health.
-
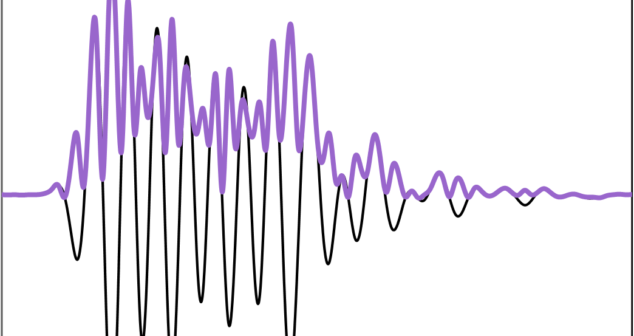
Ultrasound signal processing techniques cover a number of methods of demodulation. Pros and Cons of two that are predominant today.